The systematics of Antillean Epicrates (now Chilabothrus) are still in a chaotic state. No author has ever compared specimens of all Antillean taxa, and existing diagnoses of Antillean forms (with the exception of exsul) are at best casual. ~Sheplan & Schwartz, 1974
Distribution
There are several names that have been attributed to the Caribbean or Antilles. Historically, the word Antilles read Ant-Isles. The Greek ‘αντι , meaning opposite and Isles or Iles. They were commonly called the Caribby-Islands, Caribbies, the Cannibal Islands ( named after the ancient inhabitants) and the Camercane Islands (Davies, 1666).
The Genus Chilabothrus is distributed over the older Greater Antillean Islands (Cuba, Hispaniola, Jamaica, Puerto Rico) and Lucayan Achipelago of the West Indies. Despite close proximity and suitable habitats, the Genus Chilabothrus is absent from the newer Lesser Antilles or from mainland South-, Middle- or North America .

Historical perspective
The boas of the genus Chilabothrus (formerly Epicrates) have been described, named, redescribed and renamed over the last three centuries. While we lay out the history of each species in the accounts, we think a quick overview of the genus by species is in order. We present a succinct time line that provides: a) An historical view of the genus. b) A species level journey through time. c) The taxonomic state of the genus at present.
1725 Serpens major subflavus (Jamaican Boa)
1830 Epicrates [Genus Epicrates erected] *
1840 Epicrates angulifer (Cuban Boa)
1843 Boa inornata (Puerto Rico Boa)
1844 Chilabothrus inornatus (Puerto Rico Boa) [Genus Chilabothrus erected] **
1844 Pelophilus [Genus Pelophilus erected]
1856 Homalochilus striatus (Haitian or Fischer’s Boa) [Genus Homalochilus erected]
1861 Pelophilus fordii (Ford’s boa)
1862 Homalochilus strigilatus (Bahamas Boa)
1886 Chilabothrus (includes Homalochilus, Pelophilus, Dendrophilus and Piesigaster)
1870 Homalochilus chrysogaster (Turks/Caicos Island Boa)
1888 Chilabothus gracilis (Hispaniola Vine Boa)
1898 Epicrates monensis (Mona Island Boa)
1901 Epicrates subflavus (Jamaican Boa) [Re-described from 1725 & separated from C. inornatus]
1901 Epicrates angulifer var. striatus (Virgin Islands Boa)
1933 Epicrates inornatus granti (Virgin Islands Boa)
1935 Epicrates relicquus (Southern Bahamas Boa)
1941 Epicrates striatus fosteri (Bimini Island Boa)
1944 Epicrates exsul (Abaco Island Boa)
1974 Epicrates striatus exagistus (Tiburon Boa)
1974 Epicrates striatus warreni (Tortue Island Boa)
1974 Epicrates striatus ailurus (Cat Island Boa)
1974 Epicrates striatus mccraniei (Ragged Islands Boa)
1974 Epicrates striatus fowleri (Berry and Andros Island Boa)
1974 Epicrates fordii agametus (Mole St. Nicholas Boa)
1974 Epicrates gracilis hapalus (Tiburon Vine Boa)
1975 Epicrates chrysogaster schwartzi (Crooked/Acklins Island Boa)
1979 Epicrates fordii manototus (Ile a Cabrit Boa)
2013 Chilabothrus [Resurrected from 1844] (All boas removed from Genus Epicrates and placed in Chilabothrus)
2013 Chilabothrus strigilatus strigilatus [Elevated to full species from 1941; fosteri, fowleri, mccraniei & ailurus become subspecies]
2013 Chilabothrus striatus striatus [Retains species status ; exagistus and warreni remain subspecies]
2016 Chilabothrus argentum (Conception Island Boa)
2018 Chilabothrus schwartzi (Crooked/Acklins Island Boa) [Elevated to full species from 1975]
2021 Chilabothrus ampelophis (Hispaniolan Vineboa)
* Epicrates is from the Greek Επικρατης meaning "powerful". ** Chilabothrus is from the Greek meaning cheilos "lip", á "without", and bothros "pits".
Taxonomy
The Animal Kingdom:
Kingdom: Animalia
-Subkingdom: Bilateria
–Infrakingdom: Deuterostomia
—Phylum: Chordata
—-Subphylum: Vertebrata
—–Infraphylum: Gnathostomata
——Superclass: Tetrapoda
——-Class: Reptilia
——–Order: Squamata
———Suborder: Serpentes
———-Infraorder: Alethinophidia
———–Family: Boidae
————Genus: Chilabothrus
————-Species:
The Genus Chilabothrus was established by Duméril & Bibron . The first species which they placed in the newly formed Genus was Chilabothrus inornatus, previously described as Boa inornata by Reinhardt . Duméril & Bibron reason the erection of the new Genus as follows (translated from French):
Nostrils each opening laterally between three plates, one inter-nasal and two nasal. Lateral eyes, with vertico-elliptic pupil. Large symmetrical plates covering both first thirds of the top of the head. Point of dimples on the lips. Body scales flat, smooth; scutes Undecaudaries not divided into two parts. The Chilabothres are somehow Epicrates without labial dimples, and supra-cepialial plates in lesser number, but considerably more developed or constituting a shield that by its composition and its extent is very similar to that of the majority of Ophidians. The pieces that are part of it are a pair of internasales, a pair of fronto-nasals, a pair of prefrontal ones, a pair of sub-oculars, a frontal and four parietals. In addition, there are two plates on each side of the face nasal, fi-enae, two pre-oculars, and three or four post-oculars. This squammy vestiture of the head Chilabothres offers some resemblance to that of Liasis, the penultimate genus of the Pythonid tribe.Link to the original description by Duméril & Bibron Page: 562 Page: 563
They recognized the close resemblance of Epicrates and Chilabothrus, however, noted as well the small but significant differences between the two Genera. Yet, their conclusions were overthrown for more than a century, since Boulenger placed the West Indian species in the genus Epicrates and only recently molecular evidence revealed that mainland Epicrates are more closely related, phylogenetically, to Eunectes and that West Indian representatives form a monophyletic clade dating to the Miocene. This justified the resurrection of the genus Chilabothrus .
Different taxonomic views on Chilabothrus and the relationships within
The taxonomies based on morphological characters alone were not in all cases conclusive and some opposing concepts could not be resolved satisfactorily. For instance, Mertens believed that there are no more than three different Chilabothrus (at the time referred to as Epicrates) species present on the West Indies. He clearly saw the differences in the boas from different Islands, however he considered these as subspecies to only three boa species: Chilabothrus angulifer, C. inornatus and C. gracilis. He considered chrysogaster, striatus, strigilatus and relicquus as subspecies of C. angulifer. C. fordii, subflavus, monensis and granti as subspecies of C. inornatus. He believed that C. gracilis did not contain any subspecies. He argued that the supralabialia are not adjacent to the eyes, which he termed “the angulifer attribute” .
Sheplan and Schwartz did a very thorough analysis based purely on morphological data. They were the first to present a phylogeny for Chilabothrus reasoned directly from empirical observations. They also described several subspecies; however, the evidence to justify this was not in all cases convincing due to small sample sizes (e.g. the Chilabothrus fordii subspecies) or merely based on characters in coloration (e.g. Chilabothrus strigilatus fowleri). Nonetheless, their work was – and still is – significant and provides a very detailed and broad taxon sampling and analysis of morphological characters .
Peter Tolson pioneered a very interesting, yet today almost forgotten, study which included morphological characters as well as a skin lipid and scent gland lipid analysis. His interpretation of the results led to the second phylogeny of Chilabothrus species . Despite the fact that this phylogeny was later overthrown, it was a very interesting idea to use skin lipids and scent gland lipids, thus making the invisible differences visible. Given their role in reproduction, species recognition as well as playing roles in water retention, this analysis might be interesting for further studies on mechanisms of speciation and ecological niche fitness. Tolson used Corallus as the outgroup for his study.
Arnold Kluge conducted a similar study using 250 Epicrates (now Chilabothrus) skeletons, of which only the adult skeletal characters where scored to closely maintain comparability to Tolson’s data. Kluge used Eunectes as the outgroup vs Corallus used by Tolson. As a result, Kluge concluded “that confirmation of biochemical (Tolson) and morphological synapomorphies (Kluge) indicates a high level of congruence in the hypothesis of Epicrates (Chilabothrus) species relationships” .
Currently, however, the use of molecular techniques in phylogenetic analyses is paramount and has led in recent years to the (re)elevation of several subspecies into the rank of full species. These are Chilabothrus strigilatus , Chilabothrus granti and Chilabothrus schwartzi . In addition to this body of work, another astonishing work has been published in 2016, when the previously undescribed boa Chilabothrus argentum was scientifically analyzed and described as a full species . Most recently, another new Boa species from Hispaniola has been described Chilabothrus ampelophis, the Hispaniolan Vine Boa . In this context, it is conceivable that further molecular analyses, based on large sampling of Chilabothrus from different areas of the West Indies will lead to the description of even more new species or subspecies.
Evolution
To solve the long standing question of the evolution of Chilabothrus, Reynolds and co-workers conducted groundbreaking research using sophisticated molecular methods and taking geological and as well as morphological factors into account. Their work has led to the most complete and solid picture we have about the evolution of insular boas of the genus Chilabothrus. The current evolutionary model proposed by Reynolds et al. suggests a common ancestor for Chilabothrus and its sister group (Epicrates and Eunectes).
The common lineage split with a single dispersion event (from South America to Cuba) around ~30.0 Mya, during the Oligocene. The clade Chilabothrus has subsequently arisen about 22.0 Mya during the early Miocene. Several speciation events followed throughout the Neogene/Quaternary. This timeline of events is in temporal accordance with potential geological markers, such as the uplifting of the protoantilles which created a suitable overland route for the establishment of the genus and subsequent allopatric speciation events following dispersal to other islands.
Onary and Hsiou re-examined fossil remains which were previously analysed by different researchers and were assigned first to a viper then to Boa constrictor as well as to a genus termed Pseudoepicrates. They compared the vertebrae of the 18.5 M year old fossil snake to extant snake genera. They found that the fossil snake is a member of Chilabothrus and therefore named it Chilabothrus stanolseni comb. nov. They propose a model in which the genus probably originated in South America and dispersed from there to Cuba. From here it reached the North American mainland during the early Miocene. Their explanation suggests that the extinction event which followed in North America, was caused by climatic oscillations and thus resulting cooler temperatures at the Neogene/Quaternary boundary to which Chilabothrus was maladapted.
The West Indian populations, however, diversified throughout the Quaternary, with increased rates of dispersal between islands facilitated by abiotic events, such as a decrease in ocean level and the regular advent of hurricanes. Their research suggests that the genus originated as early as ~18.5 Mya. and is geologically and geographically consistent with the biogeographical model of Reynolds et al. . Research performed on populations of Chilabothrus chrysogaster showed the shallow genetic diversity of populations of this species . This is further support of a dispersal of very few founder animals.
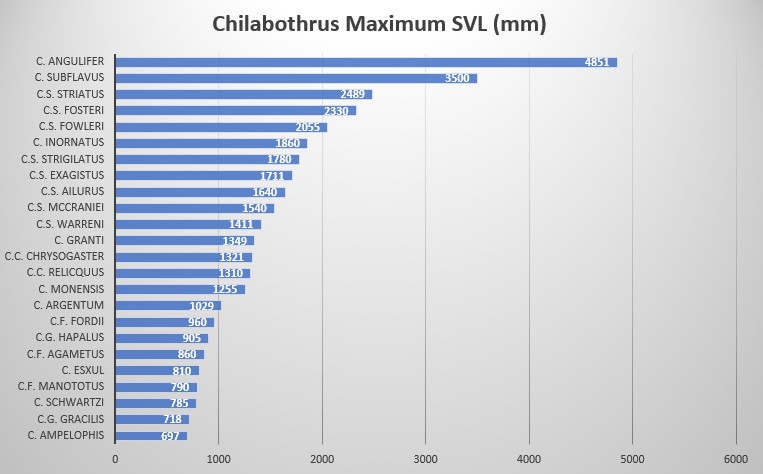
In regards to the different morphology and ethology of Chilabothrus species, a quantitative trait analyses in combination with species tree found evidence for the repeated evolution of small body size in Chilabothrus on Puerto Rico, Hispaniola and the Bahamas. These small species are comprised of microhabitat and dietary specialists derived from generalist ancestors. Other morphological characters tested in the study suggest that microhabitat specialization is also linked to increased rates of head shape diversification among specialists . Taking into consideration nanism and gigantism (e.g., C. fordii and C. angulifer), island size, prey size, selective pressures from the alteration of habitat and prey base and, in some cases, genetic predispositions to size we have a better understanding of how these boas came to be . The size difference in the genus is quite amazing, given all but one species require ectothermic prey as newborn boas. This is demonstrated by the graph below.
These results are largely in line with the body of work by Jonathan B. Losos on anoles, who showed that anole morphology is highly plastic and that these lizards adapt to new environments very quickly by changing parts of their morphology (leg lengths) over only a few generations. Losos and coworkers concluded that similar body sizes and behaviors are not a sign of close phylogenetic relationships but a result of repeated and independent specialization events driven largely by ecological opportunity coupled with intrinsic diversification potential. They coined the term “ecomorphs” for animals that don’t have a close evolutionary history, but appear similar in morphology and behavior . It should be noted that, of the twenty+ species of Chilabothrus (and Tropidophis), fifteen are single island or single bank endemics .
Species
The monumental work of Sheplan and Schwartz might be the most significant contribution to our understanding of the morphological characteristics of all Chilabothrus species, known at the time, and the variations of these species within the genus. At the time of their writing, the systematics of the Antillean forms were in a “chaotic state”. Their 86 page opus was the first of its kind as they attempted to make philogenetic sense of the entire Genus. They examined living specimens of all Antillean taxa, at the time, except C. chysogaster and C. monensis. Their 1974 publication, Hispaniolan Boas of the Genus Epicrates (Serpentes, Boidae) and their Antillean Relationships, can be found here. (It begins at page 57).
The Genus is currently comprised of 14 species . These are:
- Chilabothrus ampelophis – The Hispaniolan Vineboa, a newly described boa, is found in a very small area on the south east portion of the Dominican Republic. Restricted and threatened habitat make this boa a priority for conservation.
- Chilabothrus angulifer – The Cuban Boa is the largest of the Chilabothrus species and occurs exclusively on the Island of Cuba and adjacent islets. It has been kept by private individuals as well as in zoological collections for decades.
- Chilabothrus argentum – The Silver Boa is a recently described species of Chilabothrus. Critically endangered, occurring only on a tiny island in the West Indies.
- Chilabothrus chrysogaster – The Turks and Caicos Boa occurs in two subspecies on the Turks and Caicos islands (Chilabothrus c. chrysogaster) and on Great Inagua Island (Chilabothrus c. relicquus). It is has been kept and bred in terraria for more than 40 years.
- Chilabothrus exsul – The Abaco Island Boa occurs only on Abaco Island and is threatened by habitat loss, road kills and introduced predators. A very small population still exists in terraria.
- Chilabothrus fordii – Ford’s Boa occurs on the Island of Hispaniola. Three subspecies have been described. Commonly kept by West Indian Boa afficionados worldwide.
- Chilabothrus gracilis – The Vine Boa is one of four Boa species native to the Island of Hispaniola. This slender predator of Anoles used to be imported in large numbers and occasionally bred, but its population in captivity has declined.
- Chilabothrus granti – The Virgin Island Boa occurs on the US Virgin Islands and British Virgin Islands and in Puerto Rico. It has been kept and bred at the Toledo Zoo and is still highly endangered, despite successful conservation programs.
- Chilabothrus inornatus – The Puerto Rican Boa occurs only on the island of Puerto Rico and adjacent Islets. Though it was highly endangered in the past, protection measures helped to stabilize its population. It is commonly kept and bred in terraria.
- Chilabothrus monensis – The Mona Island Boa is an endangered species occurring only on the tiny Mona Island. It was kept and bred by The Toledo Zoo and very few private persons.
- Chilabothrus schwartzi – The Crooked Acklins Boa occurs only on the Crooked Acklins Islands of the Bahamas. It is most likely critically endangered.
- Chilabothrus striatus – The Haiti Boa occurs on the Island of Hispaniola. Three subspecies have been described. The nominate form is commonly kept by West Indian Boa afficionados worldwide.
- Chilabothrus strigilatus – The Bahamas Boa occurs on several Islands of the Bahamas. Five subspecies have been described, three of which are commonly kept by West Indian Boa afficionados worldwide.
- Chilabothrus subflavus – The Jamaican Boa is an endangered species occurring only on Jamaica. It is threatened by habitat loss and introduced predators. A self sustaining captive population exists, originating mainly from zoo stock.
Biology of Chilabothrus
The species of the Genus Chilabothrus vary greatly in body size. While several specimens above 4 meters in length have been documented for the largest species, the Cuban Boa Chilabothrus angulifer , and the maximum size has been described to reach 6.40 meters by Gundlach Cited in , the smallest members of the genus, Chilabothrus monensis, C. granti, C. fordii, C. gracilis barely reach one meter in length . The ecology and ethology of the different Chilabothrus species are highly diversified. We summarized the biological aspects of the members of this genus in the table below.
The table is largely based on . Additional data and a few changes in the data from Reynolds et al. are from the following sources as well as our own observations.
In the table above, we did not include behavioral traits, because they are often difficult to quantify. However it is certainly an interesting approach to take these into account as they must be genetically coded. Significant differences do exist between e.g., Chilabothrus striatus and C. inornatus: the former are so reluctant to bite that locals on Hispaniola even call them “sleepy snake”, in contrast to the latter which is more attentive and high strung – too often much to the surprise of the keeper.
All Chilabothrus are nocturnal. Chilabothrus species become active at dusk and are active foragers, while during the day they often are found thermoregulating. A picture series on facebook showed a Chilabothrus chrysogaster foraging and consuming a frog during daytime. Reynolds and Niemiller report an anecdote where a Chilabothrus chrysogaster was found to have recently consumed a Turks and Caicos Curly-tailed Lizard (Leiocephalus psammodromus) at 0935 h on 8 August 2008 . These two anecdotes challenge the view of Chilabothrus as strictly nocturnal foragers but indicate that islanders grasp opportunities when they appear.
Many animal species, from cats to insects to lizards and snakes, display ontogenetic color change (OCC) from juvenile to adult. Several biological reasons for the evolutionary significance of ontogenetic color change have been suggested . The origin of ontogenetic color change in Chilabothrus remains elusive. It is unclear whether the Last Common Ancestor (LCA) of all Chilabothrus species displayed this trait and it was in several lineages lost, or vice versa, ontogenetic color change evolved independently in several lineages. The biological necessity of color change from juvenile to adult is unknown for the different Chilabothrus species.
The number of young produced per litter as well as the energy invested per reproductive event (signified in the relative clutch mass) is another interesting factor to investigate when trying to reconstruct the LCA. We consider data from captive breedings as valuable to answer this question and are open to share and collaborate where possible.
Ritualized male to male combat vs. other mating strategies are highly interesting to investigate, since they have a direct impact on reproduction and speciation. In this respect, it should be added that the aforementioned Puerto Rican Boa is by no means aggressive towards conspecific males during breeding season but forms mass matings, similar to the breeding balls observed in South American anacondas (Eunectes) . In contrast, the behavior of male Chilabothrus strigilatus and C. subflavus, which (in terraria) might combat and cause serious injury or even death to the opponent.
Chilabothrus species in vivaria
Chilabothrus species have a long history in vivaria. For instance, Brehm obtained three Chilabothrus striatus from Cap Haitien which he kept in captivity . West Indian herpetofauna was exported to Europe frequently before WWII. However, in the second half of the last century, the genus Chilabothrus received greater popularity in America than in Europe as can be seen from price lists and memories of elder herpers as well as publications. Despite this imbalance in attention, several important successes have been made also by European breeders as publications demonstrate
Conservation
Detailed analyses of the conservation status exist for nine of the 14 Chilabothrus species on the IUCN website. The reports categorize the Cuban Boa (Chilabothrus angulifer) as near threatened (NT), the Conception Bank Silver Boa (C. argentum) is critically endangered (CR). The Northern Bahamas Boa (C. exsul) is categorized vulnerable (VU), the Virgin Islands Boa (C. granti) is listed as endangered (EN), the Puerto Rican Boa (C. inornatus) as least concern (LC). The Mona Island Boa (C. monensis) is listed as endangered (EN) . The Hispaniolan Boa (C. striatus) and the Bahamian Boa (C. strigilatus) are considered least concern (LC) whereas the Jamaican Boa (C. subflavus) is listed as vulnerable (VU). Population trend analyses were performed for C. argentum, C. exsul, C. striatus and C. strigilatus. These concluded that only C. striatus has a stable population trend, whereas the populations of the other species are decreasing.