GENTLEMEN, The reptiles have been comparatively neglected by recent zoologists, perhaps on account of the popular prejudices against this interesting and curious class of animals which Linneaus designates "Animalia pessima tetra nuda." It is only necessary to overcome these prejudices, and to examine them even superficially, and we cannot but be struck with the beauty of their colours, the wonderful nature of their structure, and the peculiarities of their habits and manners. Indeed I do not know any class of animals better calculated to excite the wonder and astonishment of a student of nature.
~John Edward Gray, Esq. FGS. &c. (To the Editors of the Annals of Philosophy , British Museum, July 12, 1825) The Boas – well known snakes? The term “Boa” is well known in the general public. Prominent members of this snake lineage are Boa constrictor or the Anaconda – snakes that are associated with adjectives such as big or colossal . Contrary to this, surprisingly little knowledge is present in the general public about the Superfamily as a whole and its diverse lineages and members. In their recent checklist, Reynolds and Henderson recognized a total of 66 species and 33 subspecies of boid snakes. These are distributed among 6 Families, containing 14 Genera Ungaliophis ) Eryx /Gongylophis ) that reach sizes well below one meter in length. Most members of the lineage reach sizes of around 2 meters or less in total length, which is contrary to the public sentiment of boas as giant constrictors.
Unifying anatomical characters that differentiate Booids from other snake lineages So what makes a snake a boa? The unifying characteristics Superfamily Booidae stem from the shared evolutionary history and thus relatedness. This common evolutionary history and relatedness is reflected on a molecular level in the DNA of the different species, as well as in a set of unifying morphological characters – size is not among them! These are: hind limb vestiges present as a set of spurs on either side of the vent, a moderately to well developed left lung, absent tracheal lung and, in females, both oviducts are well developed. Most species have cranial heat receptors
Several of these anatomical characters are shared with pythons, which is why pythons and boas have been considered to be closely related. Differences between Booidea and Pythonoidea are the lack of postfrontal bones and premaxillary teeth in the Boa lineage. Almost all species of Boids (the enigmatic Calabaria reinhardtii being the exception) are live bearing, whereas all pythons are exclusively egg laying. More indicative of the loose relationship are, however, the genetic differences. Research showed that Booidea and Pythonoidea are not as closely related as previously assumed.
In comparison, pythons and boas are two globally distributed and distantly related snake lineages which underwent radiations, resulting in remarkable phenotypic and ecological diversity. A recent study tested whether pythons, boas and their relatives have evolved convergent phenotypes when displaying similar ecology. The study concluded that the two groups display strong and widespread convergence in cases where they occupy equivalent ecological niches and that the history of phenotypic evolution strongly matches the history of ecological diversification. Thus suggesting a coupling of both processes, resulting in replicated adaptive radiation in both groups. Presumably strong selective pressures related to habitat-use have driven convergent evolution in phenotypes, resulting in a similar ecomorphology
The Booidea are still being researched and several surprising facts came to light only recently. These include that the genus Calabaria belongs to the Booidea Superfamily, not to the Pythonoidea . Tropidophis and it’s allies form a unique lineage not included in the Booidea . The Genus Chilabothrus was resurrected, several species were elevated from the rank of subspecies….the list goes on.
Taxonomy and zoological phylogenies in the molecular age Taxonomists used, until the early to mid 1980’s of the last century, exclusively meristic characters to differentiate species e.g.
It was the readily available DNA sequencing, insights into molecular evolution and models based on these insights, as well as other technologies associated with it, that motivated researchers to start to review phylogenetic concepts. These first molecular phylogenies had (like everything in science) some pitfalls and many were later refuted or refined. Science is a continuously ongoing process. Some of the difficulties that are related to molecular phylogenies are: choice of genes on which the phylogeny is based upon, choice of model/technology to calculate the phylogeny, too small sample size (based on few animals’ DNA), too few datapoints, too many datapoints etc. Even if, from today’s point of view, an old molecular phylogeny might look primitive these paved the way for today’s more sophisticated models.
Replicability, iterative improvement, inclusion of new data and challenging of hypotheses are some of the core principles of science and differentiate it from religious beliefs. With today’s mature state and easy applicability of molecular biological assays, most researchers rely on molecular data to analyze the differences and similarities in specific genetic or genomic sequences, and thus define relationships within animal groups and draw conclusions about evolutionary history (e.g.
Evolution of Boid snakes Molecular phylogenetic analyses clarified that the superfamily Booidea is a monophyletic group Boa, Chilabothrus, Corallus, Epicrates, Eunectes ) accounts for the highest number of species.
The Boidae emerged in the mid Cretaceous period about 145 million years ago (Mya) booidea have been discovered in North America and, most recently, in Europe. The German town of Messel hosts the biggest fossil snake assemblage discovered to date. The fossils are from the early–middle Eocene, some ~ 48 Mya. Recently researchers re-investigated Eoconstrictor fischeri and concluded that this snake was a medium- to large-sized snake (total body length ~200 cm), similar to the extant Chilabothrus inornatus . The general shape of the skull of Eoconstrictor is remarkably similar to that of neotropical boas, especially Boa constrictor . In regards to the behavior of the early boas, the researchers found that Eoconstrictor fischeri spent considerable time on the ground. Two other small Messel booids Rieppelophis ermannorum and Rageryx schmidi are inferred to be ground-dwelling forms. Messelophis variatus , a Tropidophiine snake in contrast was largely arboreal
A study investigating the Karyotypes of different Boid species (seven species and three subspecies comprised the study), found three subspecies of Boa , two species of Eunectes and three species of Epicrates that exhibit a diplooid chomosome set of 36 chromosomes. For the first time, it was shown that Corallus hortulana held a totally different karyotype than the rest of the Boidae family, a diploid set of 40 chromosomes with a greater number of macrochromosomes. Chromosomal mapping of telomeric sequences revealed the presence of interstitial telomeric sites (ITSs). The researchers concluded that chromosomal rearrangements have contributed to the diversification of the boid snakes and that ITSs are common in basal and derived lineages within the suborder Serpentes
A follow up study investigated Boid snake karyotype evolution and its mechanisms. It showed that the family Boidae differs from other families by presenting the greatest chromosomal diversity among the Booidea families. The karyotypes in this group range from 2n = 36 to 2n = 44. Most snake lineages have a set of 2n = 36 chromosomes (16 macro and 20 microchromosomes). This is considered a plesiomorphic feature for the suborder serpentes. Notably, the karyotype of the Genus Corallus differs from its sister group, the clade Eunectes , Epicrates , and Chilabothrus (all species with 2n = 36) in that the Corallus lineage exhibits two karyotype configurations, the 2n = 40 (C. hortulana, C. grenadensis ) and 2n = 44 chromosomes (C. caninus ). Corallus shared the most recent common ancestor with its sister lineages until the early Paleogene. The study proposes that the two karyotype configurations found in the Corallus lineage arose in the middle of the Paleogene/Eocene from centric fissions in all meta and submetacentric chromosomes
The question of sex chromosome evolution has been intensively studied in different vertebrate classes. Until recently it was assumed that all snakes possess ZW sex chromosomes
Exceptional in the lineage in terms of karyotype are the Corallus species, as was demonstrated by the work of Viana et al . They showed that XY chromosomes in Corallus caninus underwent fission events, which gave rise to four small acrocentric chromosomes. However, the researchers point out that it is uncertain whether a functional XY sex chromosome system exists in the C. caninus lineage or if a multiple XY system evolved through this fission event Corallus compared to other snakes from the boid lineage.
Members of the Booidea The Families and Genera in the Booidea according to
Calabariidae (Calabaria ) Sanziniidae (Sanzinia and Acrantophis ) Charinidae (subfamilies Charininae (Charina and Lichanura ) and Ungaliophiinae (Exiliboa and Ungaliophis )) Erycidae (Eryx ) Candoiidae fam. nov. (Candoia ) Boidae (Boa Corallus Eunectes Epicrates Chilabothrus A short history of Boidae Here we present the Class, Family and each genus, their creators and how the genus’ are assembled to form Boidae. Added are additional terms we think important to the study of our selection of Boas. See the Evolutionary Trees below for a species level view within the genus.
1755 Herpetology 1758 Boa Linnaeus [215 ] [216 ]1768 Constrictor 107 ]1768 Reptilia 20 ] [21 ] [22 ] [23 ]1803 Corallus 257 ] [258 ]1824 Xiphosoma 1830 Serpentes 1830 Epicrates Wagler [165 ] [166 ] [167 ] [168 ]1830 Eunectes 165 ] [166 ] [167 ] [168 ]1842 Boidae Gray [42 ] [43 ] [44 ] [45 ] [46 ]1844 Chilabothrus 563 ]1844 Pelophilus 524 ]1856 Homalochilus Fischer [101 ]
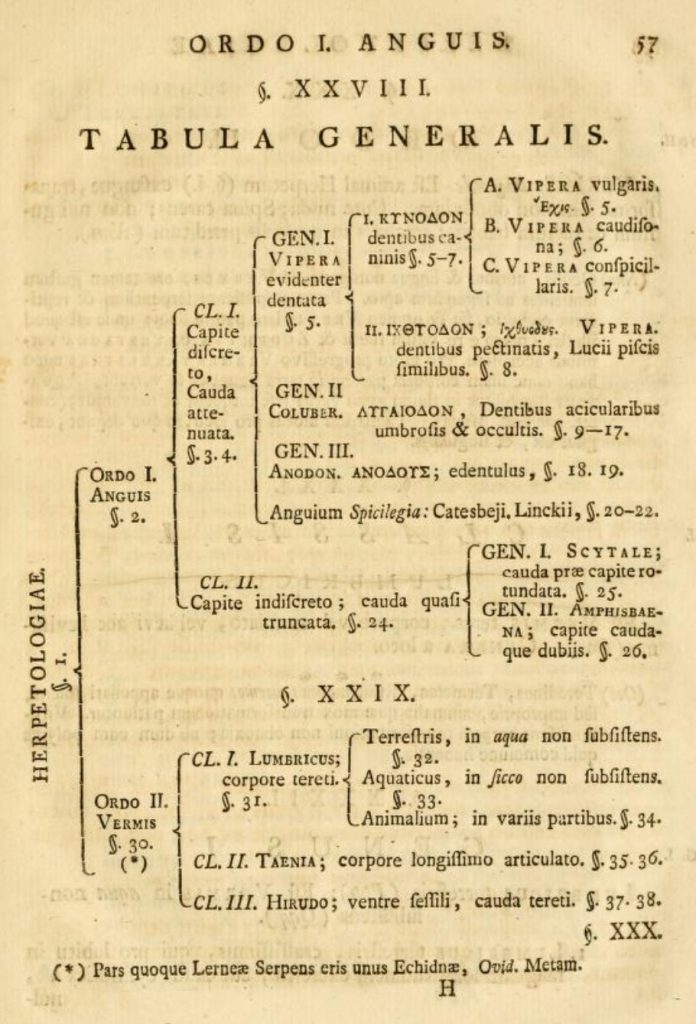
Herpetology according to Klein, 1755:
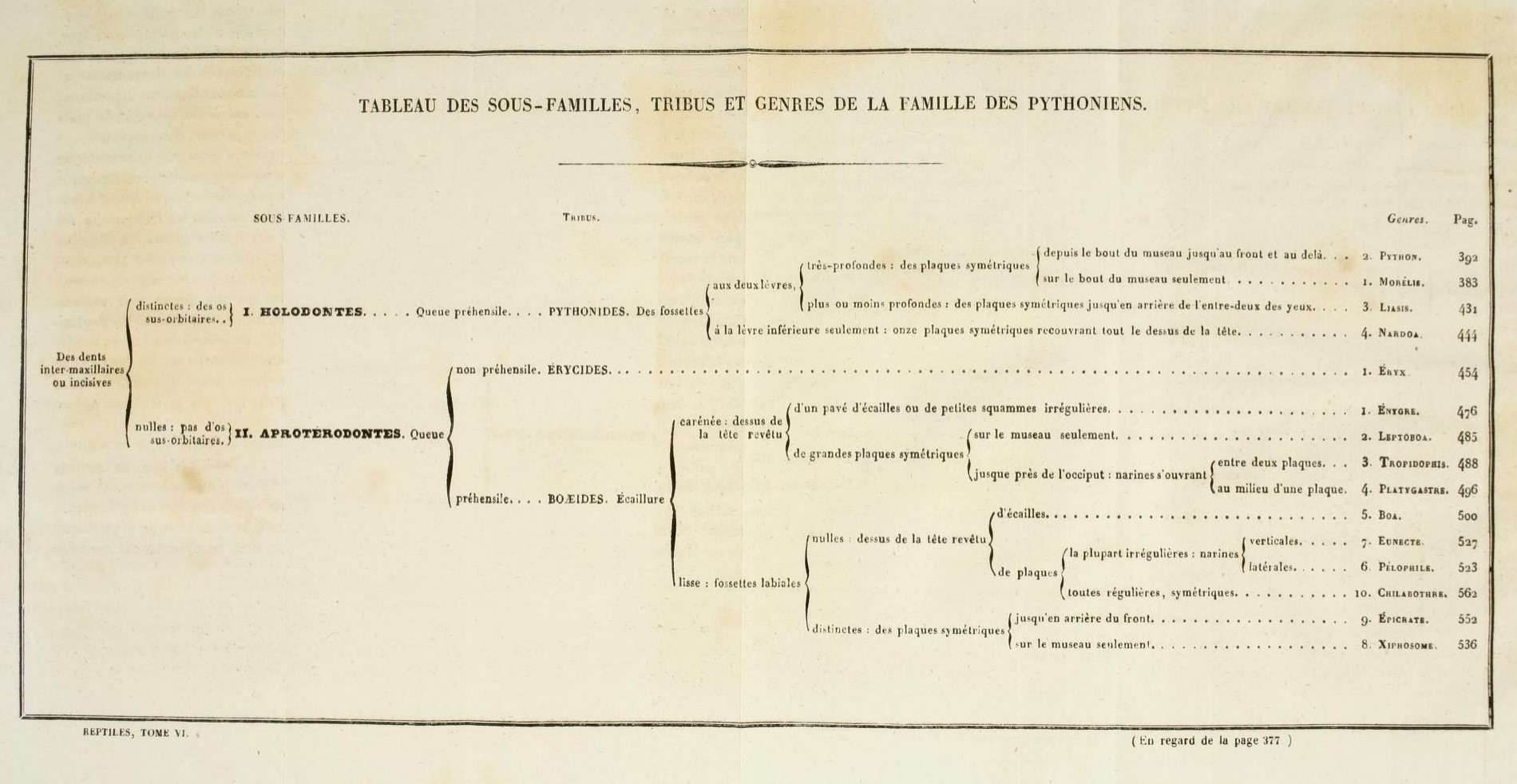
The Boidae family as depicted by Dumeril & Bibron, 1844:
Dumeril & Bibron, 1844. The Boa tree expanded from the table above.
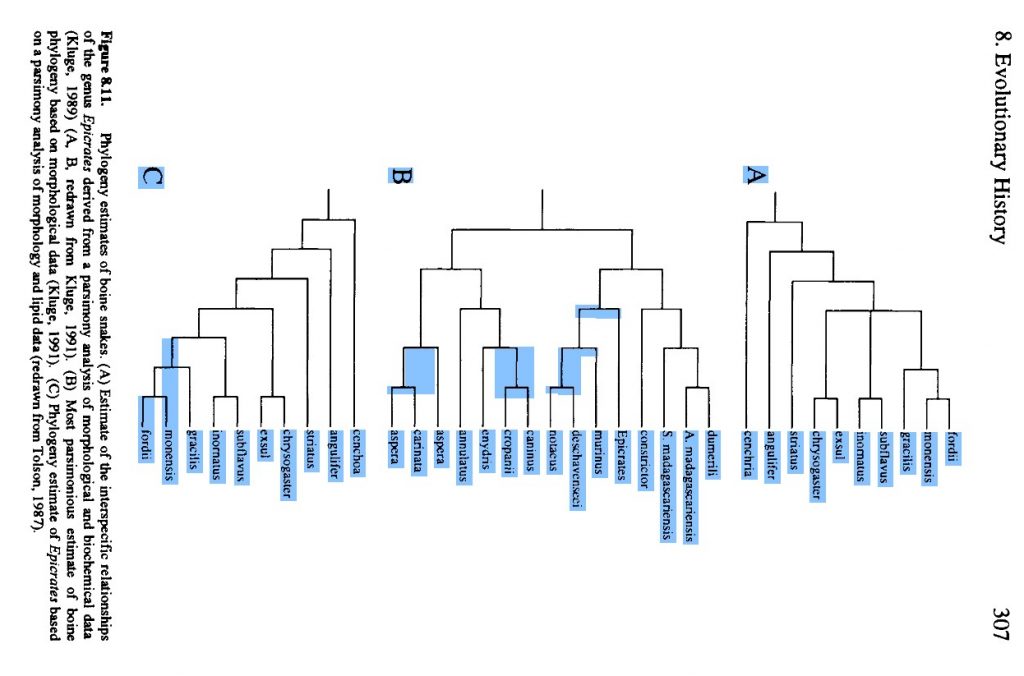
Dumeril & Bibron, 1844. The species tree in 1988:
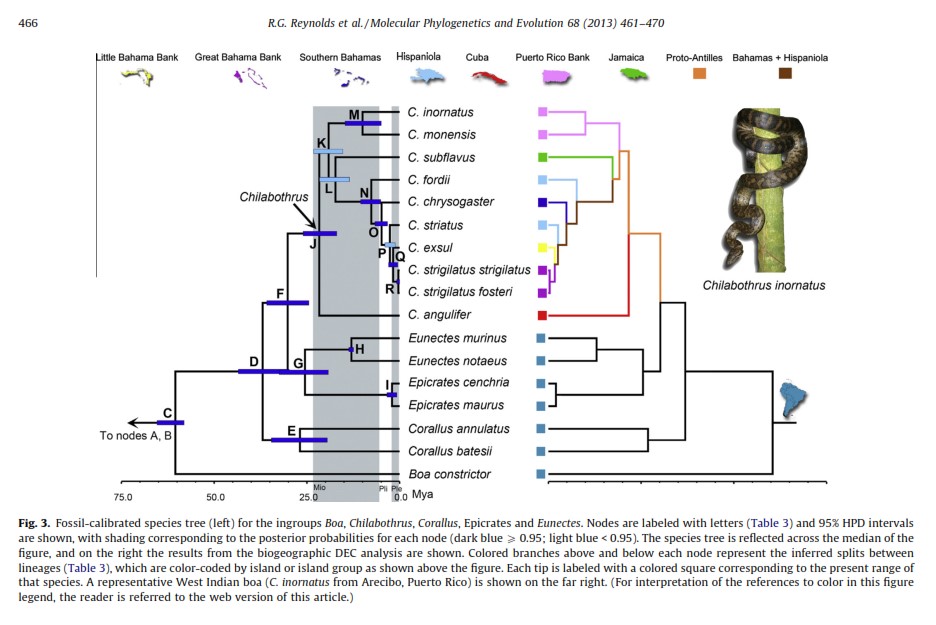
Evolutionary Relationships, 1988, Crothers The Species Tree, based on fossil evidence, in 2013:
Fossil calibrated Species Tree, 2013, Reynolds et al. * * Currently, additional genetic testing is underway and the results will provide a better understanding of the phylogeny; these results will be available later in 2021 (Reynolds pers. comm). The 2013 web version of the table above is here .
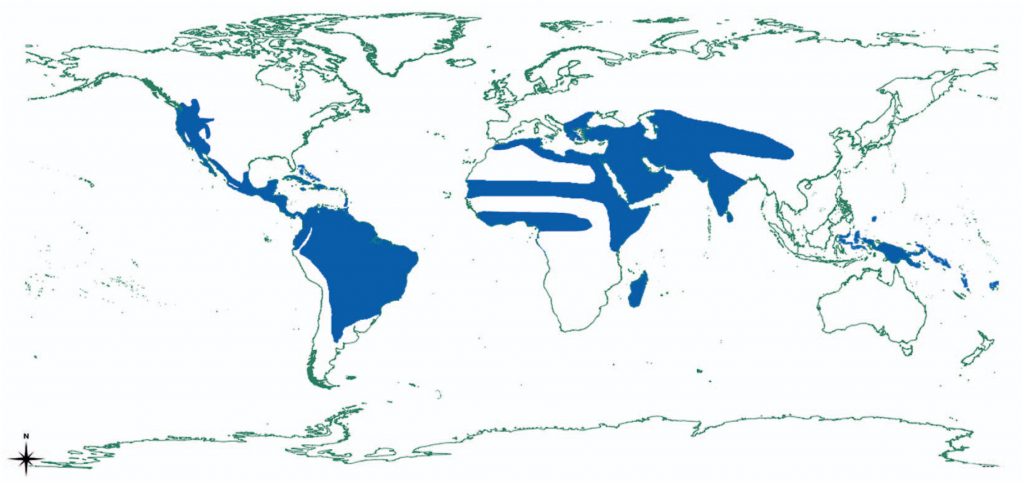
Occurrence & Biology The geographic distribution of the superfamily is large. Members of the superfamily have successfully radiated in Europe (Eryx ), Africa (Eryx and Calabaria ), Madagascar (Acranthophis and Sanzinia ), Asia (Eryx ), Asia-Pacific (Candoia ), North America (Boa, Charina and Lichanura ) Middle and South America (Exiliboa and Ungaliophis, Boa , Corallus , Eunectes , Epicrates , and Chilabothrus ). Geographically, the boas have their biggest radiaton success in Middle and South America (including the West Indies), where the majority of the species occurs. They are, however, absent from Australia and North Asia as well as from the polar regions Loxocemus bicolor . The latter being an uncertainty in terms of its phylogenetic placement in the Superfamily Pythonoidea.
Distribution of the Booidea according to Reynolds and Henderson 2018 The superfamily Booidea successfully conquered a vast diversity of habitats, from deserts (e.g. Lichanura ) to rain (e.g. Corallus batesii and C. caninus ) and cloudforests (e.g. Ungaliophis ) to Mangroves (Corallus ruschenbergerii ), from swampland (e.g. Eunectes ) to savannahs (e.g. Eryx ) and every habitat in between. With regards to the ecology, the lineage is likewise diverse, with ambush predators like Corallus species or active hunters like Chilabothrus. Harrington and colleagues analyzed the life styles of different snake families in regards to arboreality. Arboreality presents a major shift in lifestyle and is thus interesting to investigate across families. They found that 46% of boa species are arboreal (36% primarily arboreal and 64% semi arboreal). Therefore the Boidae are the second most arboreal snake family, after the Pareidae Chilabothrus gracilis ). Strategies for breeding and survival are likewise diverse and range from litters of very few young (Chilabothrus angulifer, C. fordii, C. gracilis, Lichanura trivirgata, Ungaliophis, Sanzinia ) to large litters of more than 50 young (Chilabothrus strigilatus , Eunectes murinus, Boa constrictor ). The neonate sizes differ even among closely related species (e.g. Chilabothrus angulifer (203.6 g) vs. Chilabothrus gracilis (2.0 g)).
Booidea on the West Indies Present in the West Indies are only species of the Family Boidae from the Genera Boa Corallus Eunectes Epicrates Chilabothrus Chilabothrus is endemic to the West Indies, whereas the other Genera are present also in South America and Middle America. Contrasting to the situation of genera is the species specific situation. Most of the boa species in the West Indies are true endemists and occur only on some of the islands. In a general overview article Tolson and Henderson found that of 120 snake species (representing six families and 20 genera) which inhabit the West Indies, 115 (95.8%) are endemic to the region
Spectacular image of the Virgin Islands (bottom) north to Hispaniola and the Turks/Caicos to the East. Photo credit unknown. An exception are the islands of Trinidad and Tobago, which harbor the species Boa constrictor Corallus ruschenbergerii , Epicrates maurus Eunectes murinus .
Overview map of West Indian Boas Each flag represents a single species or subspecies and the location of the flag represents the type location as originally described. Click on a flag to see the species occurring there and then on the link to get to the species account.
Continue to the Genus Boa
Citations
{2129430:SWS6AK9A};{2129430:7BDJ7ZG6};{2129430:MEBZP7NS};{2129430:BI4THB59};{2129430:MWAJWBEI},{2129430:WPYXP4KX};{2129430:4WUMTCH6};{2129430:P77ZY57F},{2129430:P79TW99Q},{2129430:P86AEZMH},{2129430:E7QA9IZV},{2129430:K7ZY4IFP};{2129430:QZMG73I2};{2129430:6F7DJWHU},{2129430:Y7SA4KVW},{2129430:7XX3SZSC},{2129430:FJSP7BEF};{2129430:79HAVRJZ},{2129430:UVGUHD5A},{2129430:VC3XU4NX},{2129430:ENK5PLHN};{2129430:TG752CN3},{2129430:LBFXPYAI};{2129430:P77ZY57F},{2129430:SWS6AK9A};{2129430:I7AINDKS};{2129430:P3X224ZR};{2129430:SHGCJE3C};{2129430:6I2NMGSM},{2129430:FVPA9DK4};{2129430:IXTGUYPP};{2129430:SHGCJE3C};{2129430:TG752CN3};{2129430:SWS6AK9A};{2129430:99QGBMTB};{2129430:VIMCTQSE};{2129430:C7NZ4QN9}
apa
author
asc
no
10 %7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3A%22zotpress-89eac4b5f7a3550805d384acb9bab7d2%22%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%227BDJ7ZG6%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bogert%22%2C%22parsedDate%22%3A%221968%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EBogert%2C%20C.%20M.%20%281968%29.%20The%20variations%20and%20affinities%20of%20the%20dwarf%20boas%20of%20the%20genus%20Ungaliophis.%20%3Ci%3EAMERICAN%20MUSEUM%20NOVITATES%3C%5C%2Fi%3E%2C%20%3Ci%3E2340%3C%5C%2Fi%3E%2C%201%26%23x2013%3B26.%20%5C%2Fz-wcorg%5C%2F.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20variations%20and%20affinities%20of%20the%20dwarf%20boas%20of%20the%20genus%20Ungaliophis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charles%20M.%22%2C%22lastName%22%3A%22Bogert%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221968%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-12T21%3A13%3A55Z%22%7D%7D%2C%7B%22key%22%3A%22QZMG73I2%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Burbrink%20and%20Pyron%22%2C%22parsedDate%22%3A%222008-04-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EBurbrink%2C%20F.%20T.%2C%20%26amp%3B%20Pyron%2C%20R.%20A.%20%282008%29.%20The%20Taming%20of%20the%20Skew%3A%20Estimating%20Proper%20Confidence%20Intervals%20for%20Divergence%20Dates.%20%3Ci%3ESystematic%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E57%3C%5C%2Fi%3E%282%29%2C%20317%26%23x2013%3B328.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1080%5C%2F10635150802040605%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1080%5C%2F10635150802040605%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Taming%20of%20the%20Skew%3A%20Estimating%20Proper%20Confidence%20Intervals%20for%20Divergence%20Dates%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frank%20T.%22%2C%22lastName%22%3A%22Burbrink%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Alexander%22%2C%22lastName%22%3A%22Pyron%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222008-04-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1080%5C%2F10635150802040605%22%2C%22ISSN%22%3A%221076-836X%2C%201063-5157%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Facademic.oup.com%5C%2Fsysbio%5C%2Farticle%5C%2F57%5C%2F2%5C%2F317%5C%2F1626758%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-10-05T11%3A03%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22BI4THB59%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Esquerr%5Cu00e9%20and%20Keogh%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EEsquerr%26%23xE9%3B%2C%20D.%2C%20%26amp%3B%20Keogh%2C%20J.%20S.%20%282016%29.%20Parallel%20selective%20pressures%20drive%20convergent%20diversification%20of%20phenotypes%20in%20pythons%20and%20boas.%20%3Ci%3EEcology%20Letters%3C%5C%2Fi%3E%2C%20%3Ci%3E19%3C%5C%2Fi%3E%287%29%2C%20800%26%23x2013%3B809.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fele.12620%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1111%5C%2Fele.12620%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Parallel%20selective%20pressures%20drive%20convergent%20diversification%20of%20phenotypes%20in%20pythons%20and%20boas%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Esquerr%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20Scott%22%2C%22lastName%22%3A%22Keogh%22%7D%5D%2C%22abstractNote%22%3A%22Pythons%20and%20boas%20are%20globally%20distributed%20and%20distantly%20related%20radiations%20with%20remarkable%20phenotypic%20and%20ecological%20diversity.%20We%20tested%20whether%20pythons%2C%20boas%20and%20their%20relatives%20have%20evolved%20convergent%20phenotypes%20when%20they%20display%20similar%20ecology.%20We%20collected%20geometric%20morphometric%20data%20on%20head%20shape%20for%201073%20specimens%20representing%20over%2080%25%20of%20species.%20We%20show%20that%20these%20two%20groups%20display%20strong%20and%20widespread%20convergence%20when%20they%20occupy%20equivalent%20ecological%20niches%20and%20that%20the%20history%20of%20phenotypic%20evolution%20strongly%20matches%20the%20history%20of%20ecological%20diversi%5Cufb01cation%2C%20suggesting%20that%20both%20processes%20are%20strongly%20coupled.%20These%20results%20are%20consistent%20with%20replicated%20adaptive%20radiation%20in%20both%20groups.%20We%20argue%20that%20strong%20selective%20pressures%20related%20to%20habitat-use%20have%20driven%20this%20convergence.%20Pythons%20and%20boas%20provide%20a%20new%20model%20system%20for%20the%20study%20of%20macro-evolutionary%20patterns%20of%20morphological%20and%20ecological%20evolution%20and%20they%20do%20so%20at%20a%20deeper%20level%20of%20divergence%20and%20global%20scale%20than%20any%20well-established%20adaptive%20radiation%20model%20systems.%22%2C%22date%22%3A%2207%5C%2F2016%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1111%5C%2Fele.12620%22%2C%22ISSN%22%3A%221461023X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdoi.wiley.com%5C%2F10.1111%5C%2Fele.12620%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222021-01-13T19%3A10%3A53Z%22%7D%7D%2C%7B%22key%22%3A%22IXTGUYPP%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gamble%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EGamble%2C%20T.%2C%20Castoe%2C%20T.%20A.%2C%20Nielsen%2C%20S.%20V.%2C%20Banks%2C%20J.%20L.%2C%20Card%2C%20D.%20C.%2C%20Schield%2C%20D.%20R.%2C%20Schuett%2C%20G.%20W.%2C%20%26amp%3B%20Booth%2C%20W.%20%282017%29.%20The%20Discovery%20of%20XY%20Sex%20Chromosomes%20in%20a%20Boa%20and%20Python.%20%3Ci%3ECurrent%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E27%3C%5C%2Fi%3E%2814%29%2C%202148-2153.e4.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cub.2017.06.010%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cub.2017.06.010%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Discovery%20of%20XY%20Sex%20Chromosomes%20in%20a%20Boa%20and%20Python%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tony%22%2C%22lastName%22%3A%22Gamble%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Todd%20A.%22%2C%22lastName%22%3A%22Castoe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stuart%20V.%22%2C%22lastName%22%3A%22Nielsen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jaison%20L.%22%2C%22lastName%22%3A%22Banks%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daren%20C.%22%2C%22lastName%22%3A%22Card%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Drew%20R.%22%2C%22lastName%22%3A%22Schield%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gordon%20W.%22%2C%22lastName%22%3A%22Schuett%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Warren%22%2C%22lastName%22%3A%22Booth%22%7D%5D%2C%22abstractNote%22%3A%22For%20over%2050%20years%2C%20biologists%20have%20accepted%20that%20all%20extant%20snakes%20share%20the%20same%20ZW%20sex%20chromosomes%20derived%20from%20a%20common%20ancestor%20%5B1%5Cu20133%5D%2C%20with%20different%20species%20exhibiting%20sex%20chromosomes%20at%20varying%20stages%20of%20differentiation.%20Accordingly%2C%20snakes%20have%20been%20a%20well-studied%20model%20for%20sex%20chromosome%20evolution%20in%20animals%20%5B1%2C%204%5D.%20A%20review%20of%20the%20literature%2C%20however%2C%20reveals%20no%20compelling%20support%20that%20boas%20and%20pythons%20possess%20ZW%20sex%20chromosomes%20%5B2%2C%205%5D.%20Furthermore%2C%20phylogenetic%20patterns%20of%20facultative%20parthenogenesis%20in%20snakes%20and%20a%20sex-linked%20color%20mutation%20in%20the%20ball%20python%20%28Python%20regius%29%20are%20best%20explained%20by%20boas%20and%20pythons%20possessing%20an%20XY%20sex%20chromosome%20system%20%5B6%2C%207%5D.%20Here%20we%20demonstrate%20that%20a%20boa%20%28Boa%20imperator%29%20and%20python%20%28Python%20bivittatus%29%20indeed%20possess%20XY%20sex%20chromosomes%2C%20based%20on%20the%20discovery%20of%20male-speci%5Cufb01c%20genetic%20markers%20in%20both%20species.%20We%20use%20these%20markers%2C%20along%20with%20transcriptomic%20and%20genomic%20data%2C%20to%20identify%20distinct%20sex%20chromosomes%20in%20boas%20and%20pythons%2C%20demonstrating%20that%20XY%20systems%20evolved%20independently%20in%20each%20lineage.%20This%20discovery%20highlights%20the%20dynamic%20evolution%20of%20vertebrate%20sex%20chromosomes%20and%20further%20enhances%20the%20value%20of%20snakes%20as%20a%20model%20for%20studying%20sex%20chromosome%20evolution.%22%2C%22date%22%3A%2207%5C%2F2017%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.cub.2017.06.010%22%2C%22ISSN%22%3A%2209609822%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS096098221730711X%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-12-04T15%3A49%3A44Z%22%7D%7D%2C%7B%22key%22%3A%22FJSP7BEF%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gilbert%20et%20al.%22%2C%22parsedDate%22%3A%222008-06-17%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EGilbert%2C%20M.%20T.%20P.%2C%20Drautz%2C%20D.%20I.%2C%20Lesk%2C%20A.%20M.%2C%20Ho%2C%20S.%20Y.%20W.%2C%20Qi%2C%20J.%2C%20Ratan%2C%20A.%2C%20Hsu%2C%20C.-H.%2C%20Sher%2C%20A.%2C%20Dalen%2C%20L.%2C%20Gotherstrom%2C%20A.%2C%20Tomsho%2C%20L.%20P.%2C%20Rendulic%2C%20S.%2C%20Packard%2C%20M.%2C%20Campos%2C%20P.%20F.%2C%20Kuznetsova%2C%20T.%20V.%2C%20Shidlovskiy%2C%20F.%2C%20Tikhonov%2C%20A.%2C%20Willerslev%2C%20E.%2C%20Iacumin%2C%20P.%2C%20%26%23x2026%3B%20Schuster%2C%20S.%20C.%20%282008%29.%20Intraspecific%20phylogenetic%20analysis%20of%20Siberian%20woolly%20mammoths%20using%20complete%20mitochondrial%20genomes.%20%3Ci%3EProceedings%20of%20the%20National%20Academy%20of%20Sciences%3C%5C%2Fi%3E%2C%20%3Ci%3E105%3C%5C%2Fi%3E%2824%29%2C%208327%26%23x2013%3B8332.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.0802315105%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.0802315105%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Intraspecific%20phylogenetic%20analysis%20of%20Siberian%20woolly%20mammoths%20using%20complete%20mitochondrial%20genomes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%20T.%20P.%22%2C%22lastName%22%3A%22Gilbert%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D.%20I.%22%2C%22lastName%22%3A%22Drautz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20M.%22%2C%22lastName%22%3A%22Lesk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%20Y.%20W.%22%2C%22lastName%22%3A%22Ho%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Qi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Ratan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.-H.%22%2C%22lastName%22%3A%22Hsu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Sher%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%22%2C%22lastName%22%3A%22Dalen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Gotherstrom%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L.%20P.%22%2C%22lastName%22%3A%22Tomsho%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Rendulic%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Packard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20F.%22%2C%22lastName%22%3A%22Campos%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%20V.%22%2C%22lastName%22%3A%22Kuznetsova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22F.%22%2C%22lastName%22%3A%22Shidlovskiy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Tikhonov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%22%2C%22lastName%22%3A%22Willerslev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Iacumin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.%22%2C%22lastName%22%3A%22Buigues%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20G.%20P.%22%2C%22lastName%22%3A%22Ericson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Germonpre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Kosintsev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22V.%22%2C%22lastName%22%3A%22Nikolaev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Nowak-Kemp%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20R.%22%2C%22lastName%22%3A%22Knight%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%20P.%22%2C%22lastName%22%3A%22Irzyk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20S.%22%2C%22lastName%22%3A%22Perbost%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20M.%22%2C%22lastName%22%3A%22Fredrikson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%20T.%22%2C%22lastName%22%3A%22Harkins%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Sheridan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22W.%22%2C%22lastName%22%3A%22Miller%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%20C.%22%2C%22lastName%22%3A%22Schuster%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222008-06-17%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.0802315105%22%2C%22ISSN%22%3A%220027-8424%2C%201091-6490%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.0802315105%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-10-24T10%3A47%3A31Z%22%7D%7D%2C%7B%22key%22%3A%22P79TW99Q%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guo%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EGuo%2C%20P.%2C%20Liu%2C%20Q.%2C%20Xu%2C%20Y.%2C%20Jiang%2C%20K.%2C%20Hou%2C%20M.%2C%20Ding%2C%20L.%2C%20Pyron%2C%20R.%20A.%2C%20%26amp%3B%20Burbrink%2C%20F.%20T.%20%282012%29.%20Out%20of%20Asia%3A%20Natricine%20snakes%20support%20the%20Cenozoic%20Beringian%20Dispersal%20Hypothesis.%20%3Ci%3EMolecular%20Phylogenetics%20and%20Evolution%3C%5C%2Fi%3E%2C%20%3Ci%3E63%3C%5C%2Fi%3E%283%29%2C%20825%26%23x2013%3B833.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2012.02.021%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2012.02.021%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Out%20of%20Asia%3A%20Natricine%20snakes%20support%20the%20Cenozoic%20Beringian%20Dispersal%20Hypothesis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peng%22%2C%22lastName%22%3A%22Guo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Qin%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yan%22%2C%22lastName%22%3A%22Xu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ke%22%2C%22lastName%22%3A%22Jiang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mian%22%2C%22lastName%22%3A%22Hou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Li%22%2C%22lastName%22%3A%22Ding%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Alexander%22%2C%22lastName%22%3A%22Pyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frank%20T.%22%2C%22lastName%22%3A%22Burbrink%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%226%5C%2F2012%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ympev.2012.02.021%22%2C%22ISSN%22%3A%2210557903%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1055790312000747%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-08-04T11%3A39%3A27Z%22%7D%7D%2C%7B%22key%22%3A%22ENK5PLHN%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hsiang%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EHsiang%2C%20A.%20Y.%2C%20Field%2C%20D.%20J.%2C%20Webster%2C%20T.%20H.%2C%20Behlke%2C%20A.%20D.%2C%20Davis%2C%20M.%20B.%2C%20Racicot%2C%20R.%20A.%2C%20%26amp%3B%20Gauthier%2C%20J.%20A.%20%282015%29.%20The%20origin%20of%20snakes%3A%20revealing%20the%20ecology%2C%20behavior%2C%20and%20evolutionary%20history%20of%20early%20snakes%20using%20genomics%2C%20phenomics%2C%20and%20the%20fossil%20record.%20%3Ci%3EBMC%20Evolutionary%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E15%3C%5C%2Fi%3E%281%29%2C%2087.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12862-015-0358-5%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12862-015-0358-5%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20origin%20of%20snakes%3A%20revealing%20the%20ecology%2C%20behavior%2C%20and%20evolutionary%20history%20of%20early%20snakes%20using%20genomics%2C%20phenomics%2C%20and%20the%20fossil%20record%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Allison%20Y%22%2C%22lastName%22%3A%22Hsiang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20J%22%2C%22lastName%22%3A%22Field%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Timothy%20H%22%2C%22lastName%22%3A%22Webster%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Adam%20DB%22%2C%22lastName%22%3A%22Behlke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthew%20B%22%2C%22lastName%22%3A%22Davis%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rachel%20A%22%2C%22lastName%22%3A%22Racicot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacques%20A%22%2C%22lastName%22%3A%22Gauthier%22%7D%5D%2C%22abstractNote%22%3A%22Background%3A%20The%20highly%20derived%20morphology%20and%20astounding%20diversity%20of%20snakes%20has%20long%20inspired%20debate%20regarding%20the%20ecological%20and%20evolutionary%20origin%20of%20both%20the%20snake%20total-group%20%28Pan-Serpentes%29%20and%20crown%20snakes%20%28Serpentes%29.%20Although%20speculation%20abounds%20on%20the%20ecology%2C%20behavior%2C%20and%20provenance%20of%20the%20earliest%20snakes%2C%20a%20rigorous%2C%20clade-wide%20analysis%20of%20snake%20origins%20has%20yet%20to%20be%20attempted%2C%20in%20part%20due%20to%20a%20dearth%20of%20adequate%20paleontological%20data%20on%20early%20stem%20snakes.%20Here%2C%20we%20present%20the%20first%20comprehensive%20analytical%20reconstruction%20of%20the%20ancestor%20of%20crown%20snakes%20and%20the%20ancestor%20of%20the%20snake%20total-group%2C%20as%20inferred%20using%20multiple%20methods%20of%20ancestral%20state%20reconstruction.%20We%20use%20a%20combined-data%20approach%20that%20includes%20new%20information%20from%20the%20fossil%20record%20on%20extinct%20crown%20snakes%2C%20new%20data%20on%20the%20anatomy%20of%20the%20stem%20snakes%20Najash%20rionegrina%2C%20Dinilysia%20patagonica%2C%20and%20Coniophis%20precedens%2C%20and%20a%20deeper%20understanding%20of%20the%20distribution%20of%20phenotypic%20apomorphies%20among%20the%20major%20clades%20of%20fossil%20and%20Recent%20snakes.%20Additionally%2C%20we%20infer%20time-calibrated%20phylogenies%20using%20both%20new%20%5Cu2018tip-dating%5Cu2019%20and%20traditional%20node-based%20approaches%2C%20providing%20new%20insights%20on%20temporal%20patterns%20in%20the%20early%20evolutionary%20history%20of%20snakes.%5CnResults%3A%20Comprehensive%20ancestral%20state%20reconstructions%20reveal%20that%20both%20the%20ancestor%20of%20crown%20snakes%20and%20the%20ancestor%20of%20total-group%20snakes%20were%20nocturnal%2C%20widely%20foraging%2C%20non-constricting%20stealth%20hunters.%20They%20likely%20consumed%20soft-bodied%20vertebrate%20and%20invertebrate%20prey%20that%20was%20subequal%20to%20head%20size%2C%20and%20occupied%20terrestrial%20settings%20in%20warm%2C%20well-watered%2C%20and%20well-vegetated%20environments.%20The%20snake%20total-group%20%5Cu2013%20approximated%20by%20the%20Coniophis%20node%20%5Cu2013%20is%20inferred%20to%20have%20originated%20on%20land%20during%20the%20middle%20Early%20Cretaceous%20%28~128.5%20Ma%29%2C%20with%20the%20crown-group%20following%20about%2020%20million%20years%20later%2C%20during%20the%20Albian%20stage.%20Our%20inferred%20divergence%20dates%20provide%20strong%20evidence%20for%20a%20major%20radiation%20of%20henophidian%20snake%20diversity%20in%20the%20wake%20of%20the%20Cretaceous-Paleogene%20%28K-Pg%29%20mass%20extinction%2C%20clarifying%20the%20pattern%20and%20timing%20of%20the%20extant%20snake%20radiation.%20Although%20the%20snake%20crown-group%20most%20likely%20arose%20on%20the%20supercontinent%20of%20Gondwana%2C%20our%20results%20suggest%20the%20possibility%20that%20the%20snake%20total-group%20originated%20on%20Laurasia.%5CnConclusions%3A%20Our%20study%20provides%20new%20insights%20into%20when%2C%20where%2C%20and%20how%20snakes%20originated%2C%20and%20presents%20the%20most%20complete%20picture%20of%20the%20early%20evolution%20of%20snakes%20to%20date.%20More%20broadly%2C%20we%20demonstrate%20the%20striking%20influence%20of%20including%20fossils%20and%20phenotypic%20data%20in%20combined%20analyses%20aimed%20at%20both%20phylogenetic%20topology%20inference%20and%20ancestral%20state%20reconstruction.%22%2C%22date%22%3A%2212%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1186%5C%2Fs12862-015-0358-5%22%2C%22ISSN%22%3A%221471-2148%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.biomedcentral.com%5C%2F1471-2148%5C%2F15%5C%2F87%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-09-01T19%3A48%3A20Z%22%7D%7D%2C%7B%22key%22%3A%2279HAVRJZ%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lee%20et%20al.%22%2C%22parsedDate%22%3A%222007%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ELee%2C%20M.%20S.%20Y.%2C%20Hugall%2C%20A.%20F.%2C%20Lawson%2C%20R.%2C%20%26amp%3B%20Scanlon%2C%20J.%20D.%20%282007%29.%20Phylogeny%20of%20snakes%20%28Serpentes%29%3A%20Combining%20morphological%20and%20molecular%20data%20in%20likelihood%2C%20Bayesian%20and%20parsimony%20analyses.%20%3Ci%3ESystematics%20and%20Biodiversity%3C%5C%2Fi%3E%2C%20%3Ci%3E5%3C%5C%2Fi%3E%284%29%2C%20371%26%23x2013%3B389.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1017%5C%2FS1477200007002290%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1017%5C%2FS1477200007002290%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phylogeny%20of%20snakes%20%28Serpentes%29%3A%20Combining%20morphological%20and%20molecular%20data%20in%20likelihood%2C%20Bayesian%20and%20parsimony%20analyses%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%20S.%20Y.%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrew%20F.%22%2C%22lastName%22%3A%22Hugall%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Robin%22%2C%22lastName%22%3A%22Lawson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20D.%22%2C%22lastName%22%3A%22Scanlon%22%7D%5D%2C%22abstractNote%22%3A%22Abstract%20The%20phylogeny%20of%20living%20and%20fossil%20snakes%20is%20assessed%20using%20likelihood%20and%20parsimony%20approaches%20and%20a%20dataset%20combining%20263%20morphological%20characters%20with%20mitochondrial%20%282693%20bp%29%20and%20nuclear%20%281092%20bp%29%20gene%20sequences.%20The%20%5Cu2018no%20common%20mechanism%5Cu2019%20%28NCMr%29%20and%20%5Cu2018Markovian%5Cu2019%20%28Mkv%29%20models%20were%20employed%20for%20the%20morphological%20partition%20in%20likelihood%20analyses%3B%20likelihood%20scores%20in%20the%20NCMr%20model%20were%20more%20closely%20correlated%20with%20parsimony%20tree%20lengths.%20Both%20models%20accorded%20relatively%20less%20weight%20to%20the%20molecular%20data%20than%20did%20parsimony%2C%20with%20the%20effect%20being%20milder%20in%20the%20NCMr%20model.%20Partitioned%20branch%20and%20likelihood%20support%20values%20indicate%20that%20the%20mtDNA%20and%20nuclear%20gene%20partitions%20agree%20more%20closely%20with%20each%20other%20than%20with%20morphology.%20Despite%20differences%20between%20data%20partitions%20in%20phylogenetic%20signal%2C%20analytic%20models%2C%20and%20relative%20weighting%2C%20the%20parsimony%20and%20likelihood%20analyses%20all%20retrieved%20the%20following%20widely%20accepted%20groups%3A%20scolecophidians%2C%20alethinophidians%2C%20cylindrophiines%2C%20macrostomatans%20%28sensu%20lato%29%20and%20caenophidians.%20Anilius%20alone%20emerged%20as%20the%20most%20basal%20alethinophidian%3B%20the%20combined%20analyses%20resulted%20in%20a%20novel%20and%20stable%20position%20of%20uropeltines%20and%20cylindrophiines%20as%20the%20second-most%20basal%20clade%20of%20alethinophidians.%20The%20limbed%20marine%20pachyophiids%2C%20along%20with%20Dinilysia%20and%20Wonambi%2C%20were%20always%20basal%20to%20all%20living%20snakes.%20Other%20results%20stable%20in%20all%20combined%20analyses%20include%3A%20Xenopeltis%20and%20Loxocemus%20were%20sister%20taxa%20%28%20%5Cufb01de%20morphology%29%20but%20clustered%20with%20pythonines%20%28%20%5Cufb01de%20molecules%29%2C%20and%20Ungaliophis%20clustered%20with%20a%20boine-erycine%20clade%20%28%20%5Cufb01de%20molecules%29.%20Tropidophis%20remains%20enigmatic%3B%20it%20emerges%20as%20a%20basal%20alethinophidian%20in%20the%20parsimony%20analyses%20%28%20%5Cufb01de%20molecules%29%20but%20a%20derived%20form%20in%20the%20likelihood%20analyses%20%28%20%5Cufb01de%20morphology%29%2C%20largely%20due%20to%20the%20different%20relative%20weighting%20accorded%20to%20data%20partitions.%22%2C%22date%22%3A%2211%5C%2F2007%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1017%5C%2FS1477200007002290%22%2C%22ISSN%22%3A%221477-2000%2C%201478-0933%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.tandfonline.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1017%5C%2FS1477200007002290%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-12-29T16%3A52%3A48Z%22%7D%7D%2C%7B%22key%22%3A%22FVPA9DK4%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Matsubara%20et%20al.%22%2C%22parsedDate%22%3A%222006%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EMatsubara%2C%20K.%2C%20Tarui%2C%20H.%2C%20Toriba%2C%20M.%2C%20Yamada%2C%20K.%2C%20Nishida-Umehara%2C%20C.%2C%20Agata%2C%20K.%2C%20%26amp%3B%20Matsuda%2C%20Y.%20%282006%29.%20Evidence%20for%20different%20origin%20of%20sex%20chromosomes%20in%20snakes%2C%20birds%2C%20and%20mammals%20and%20step-wise%20differentiation%20of%20snake%20sex%20chromosomes.%20%3Ci%3EProceedings%20of%20the%20National%20Academy%20of%20Sciences%20of%20the%20United%20States%20of%20America%3C%5C%2Fi%3E%2C%20%3Ci%3E103%3C%5C%2Fi%3E%2848%29%2C%2018190%26%23x2013%3B18195.%20%3Ca%20href%3D%27www.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.0605274103%27%3Ewww.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.0605274103%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Evidence%20for%20different%20origin%20of%20sex%20chromosomes%20in%20snakes%2C%20birds%2C%20and%20mammals%20and%20step-wise%20differentiation%20of%20snake%20sex%20chromosomes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kazumi%22%2C%22lastName%22%3A%22Matsubara%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hiroshi%22%2C%22lastName%22%3A%22Tarui%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michihisa%22%2C%22lastName%22%3A%22Toriba%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kazuhiko%22%2C%22lastName%22%3A%22Yamada%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chizuko%22%2C%22lastName%22%3A%22Nishida-Umehara%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kiyokazu%22%2C%22lastName%22%3A%22Agata%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yoichi%22%2C%22lastName%22%3A%22Matsuda%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222006%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22www.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.0605274103%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222021-11-03T20%3A40%3A04Z%22%7D%7D%2C%7B%22key%22%3A%22C7NZ4QN9%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nellis%20et%20al.%22%2C%22parsedDate%22%3A%221983%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ENellis%2C%20D.%20W.%2C%20Norton%2C%20R.%20L.%2C%20%26amp%3B%20MacLean%2C%20W.%20P.%20%281983%29.%20On%20the%20Biogeography%20of%20the%20Virgin%20Islands%20Boa%2C%20Epicrates%20monensis%20granti.%20%3Ci%3EJournal%20of%20Herpetology%3C%5C%2Fi%3E%2C%20%3Ci%3E17%3C%5C%2Fi%3E%284%29%2C%20413.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.2307%5C%2F1563600%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.2307%5C%2F1563600%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22On%20the%20Biogeography%20of%20the%20Virgin%20Islands%20Boa%2C%20Epicrates%20monensis%20granti%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%20W.%22%2C%22lastName%22%3A%22Nellis%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Robert%20L.%22%2C%22lastName%22%3A%22Norton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22William%20P.%22%2C%22lastName%22%3A%22MacLean%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2212%5C%2F1983%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.2307%5C%2F1563600%22%2C%22ISSN%22%3A%2200221511%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.jstor.org%5C%2Fstable%5C%2F1563600%3Forigin%3Dcrossref%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-24T13%3A19%3A49Z%22%7D%7D%2C%7B%22key%22%3A%227XX3SZSC%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Noonan%22%2C%22parsedDate%22%3A%222010-05-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ENoonan%2C%20J.%20P.%20%282010%29.%20Neanderthal%20genomics%20and%20the%20evolution%20of%20modern%20humans.%20%3Ci%3EGenome%20Research%3C%5C%2Fi%3E%2C%20%3Ci%3E20%3C%5C%2Fi%3E%285%29%2C%20547%26%23x2013%3B553.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1101%5C%2Fgr.076000.108%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1101%5C%2Fgr.076000.108%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Neanderthal%20genomics%20and%20the%20evolution%20of%20modern%20humans%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20P.%22%2C%22lastName%22%3A%22Noonan%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222010-05-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1101%5C%2Fgr.076000.108%22%2C%22ISSN%22%3A%221088-9051%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fgenome.cshlp.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1101%5C%2Fgr.076000.108%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-10-24T10%3A47%3A31Z%22%7D%7D%2C%7B%22key%22%3A%226F7DJWHU%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Noonan%22%2C%22parsedDate%22%3A%222005-07-22%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ENoonan%2C%20J.%20P.%20%282005%29.%20Genomic%20Sequencing%20of%20Pleistocene%20Cave%20Bears.%20%3Ci%3EScience%3C%5C%2Fi%3E%2C%20%3Ci%3E309%3C%5C%2Fi%3E%285734%29%2C%20597%26%23x2013%3B599.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1126%5C%2Fscience.1113485%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1126%5C%2Fscience.1113485%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Genomic%20Sequencing%20of%20Pleistocene%20Cave%20Bears%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20P.%22%2C%22lastName%22%3A%22Noonan%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222005-07-22%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1126%5C%2Fscience.1113485%22%2C%22ISSN%22%3A%220036-8075%2C%201095-9203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.sciencemag.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1126%5C%2Fscience.1113485%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-10-24T10%3A49%3A12Z%22%7D%7D%2C%7B%22key%22%3A%22TG752CN3%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pyron%20et%20al.%22%2C%22parsedDate%22%3A%222014-08-01%22%2C%22numChildren%22%3A7%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EPyron%2C%20R.%20A.%2C%20Reynolds%2C%20R.%20G.%2C%20%26amp%3B%20Burbrink%2C%20F.%20T.%20%282014%29.%20A%20Taxonomic%20Revision%20of%20Boas%20%28Serpentes%3A%20Boidae%29.%20%3Ci%3EZootaxa%3C%5C%2Fi%3E%2C%20%3Ci%3E3846%3C%5C%2Fi%3E%282%29%2C%20249.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.11646%5C%2Fzootaxa.3846.2.5%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.11646%5C%2Fzootaxa.3846.2.5%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Taxonomic%20Revision%20of%20Boas%20%28Serpentes%3A%20Boidae%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Alexander%22%2C%22lastName%22%3A%22Pyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Graham%22%2C%22lastName%22%3A%22Reynolds%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frank%20T.%22%2C%22lastName%22%3A%22Burbrink%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222014-08-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.11646%5C%2Fzootaxa.3846.2.5%22%2C%22ISSN%22%3A%221175-5334%2C%201175-5326%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fbiotaxa.org%5C%2FZootaxa%5C%2Farticle%5C%2Fview%5C%2Fzootaxa.3846.2.5%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-18T22%3A00%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22P86AEZMH%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Pyron%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EPyron%2C%20R.%20A.%2C%20Burbrink%2C%20F.%20T.%2C%20%26amp%3B%20Wiens%2C%20J.%20J.%20%282013%29.%20A%20phylogeny%20and%20revised%20classification%20of%20Squamata%2C%20including%204161%20species%20of%20lizards%20and%20snakes.%20%3Ci%3EBMC%20Evolutionary%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E13%3C%5C%2Fi%3E%281%29%2C%2093.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1471-2148-13-93%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1471-2148-13-93%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20phylogeny%20and%20revised%20classification%20of%20Squamata%2C%20including%204161%20species%20of%20lizards%20and%20snakes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Alexander%22%2C%22lastName%22%3A%22Pyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frank%20T%22%2C%22lastName%22%3A%22Burbrink%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20J%22%2C%22lastName%22%3A%22Wiens%22%7D%5D%2C%22abstractNote%22%3A%22Background%3A%20The%20extant%20squamates%20%28%3E9400%20known%20species%20of%20lizards%20and%20snakes%29%20are%20one%20of%20the%20most%20diverse%20and%20conspicuous%20radiations%20of%20terrestrial%20vertebrates%2C%20but%20no%20studies%20have%20attempted%20to%20reconstruct%20a%20phylogeny%20for%20the%20group%20with%20large-scale%20taxon%20sampling.%20Such%20an%20estimate%20is%20invaluable%20for%20comparative%20evolutionary%20studies%2C%20and%20to%20address%20their%20classification.%20Here%2C%20we%20present%20the%20first%20large-scale%20phylogenetic%20estimate%20for%20Squamata.%5CnResults%3A%20The%20estimated%20phylogeny%20contains%204161%20species%2C%20representing%20all%20currently%20recognized%20families%20and%20subfamilies.%20The%20analysis%20is%20based%20on%20up%20to%2012896%20base%20pairs%20of%20sequence%20data%20per%20species%20%28average%20%3D%202497%20bp%29%20from%2012%20genes%2C%20including%20seven%20nuclear%20loci%20%28BDNF%2C%20c-mos%2C%20NT3%2C%20PDC%2C%20R35%2C%20RAG-1%2C%20and%20RAG-2%29%2C%20and%20five%20mitochondrial%20genes%20%2812S%2C%2016S%2C%20cytochrome%20b%2C%20ND2%2C%20and%20ND4%29.%20The%20tree%20provides%20important%20confirmation%20for%20recent%20estimates%20of%20higher-level%20squamate%20phylogeny%20based%20on%20molecular%20data%20%28but%20with%20more%20limited%20taxon%20sampling%29%2C%20estimates%20that%20are%20very%20different%20from%20previous%20morphology-based%20hypotheses.%20The%20tree%20also%20includes%20many%20relationships%20that%20differ%20from%20previous%20molecular%20estimates%20and%20many%20that%20differ%20from%20traditional%20taxonomy.%5CnConclusions%3A%20We%20present%20a%20new%20large-scale%20phylogeny%20of%20squamate%20reptiles%20that%20should%20be%20a%20valuable%20resource%20for%20future%20comparative%20studies.%20We%20also%20present%20a%20revised%20classification%20of%20squamates%20at%20the%20family%20and%20subfamily%20level%20to%20bring%20the%20taxonomy%20more%20in%20line%20with%20the%20new%20phylogenetic%20hypothesis.%20This%20classification%20includes%20new%2C%20resurrected%2C%20and%20modified%20subfamilies%20within%20gymnophthalmid%20and%20scincid%20lizards%2C%20and%20boid%2C%20colubrid%2C%20and%20lamprophiid%20snakes.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1186%5C%2F1471-2148-13-93%22%2C%22ISSN%22%3A%221471-2148%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fbmcevolbiol.biomedcentral.com%5C%2Farticles%5C%2F10.1186%5C%2F1471-2148-13-93%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-08-04T11%3A39%3A38Z%22%7D%7D%2C%7B%22key%22%3A%22UVGUHD5A%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Reiserer%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EReiserer%2C%20R.%2C%20Schuett%2C%20G.%2C%20%26amp%3B%20Beck%2C%20D.%20%282013%29.%20Taxonomic%20reassessment%20and%20conservation%20status%20of%20the%20beaded%20lizard%2C%20Heloderma%20horridum%20%28Squamata%3A%20Helodermatidae%29.%20%3Ci%3EAmphibian%20%26amp%3B%20Reptile%20Conservation%3C%5C%2Fi%3E%2C%20%3Ci%3E7%3C%5C%2Fi%3E%2C%2074%26%23x2013%3B96.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Taxonomic%20reassessment%20and%20conservation%20status%20of%20the%20beaded%20lizard%2C%20Heloderma%20horridum%20%28Squamata%3A%20Helodermatidae%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Randall%22%2C%22lastName%22%3A%22Reiserer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gordon%22%2C%22lastName%22%3A%22Schuett%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%22%2C%22lastName%22%3A%22Beck%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2201%202013%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-02-28T10%3A03%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22SWS6AK9A%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Reynolds%20and%20Henderson%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EReynolds%2C%20R.%20G.%2C%20%26amp%3B%20Henderson%2C%20R.%20W.%20%282018%29.%20Boas%20of%20the%20World%20%28Superfamily%20Booidae%29%3A%20A%20Checklist%20With%20Systematic%2C%20Taxonomic%2C%20and%20Conservation%20Assessments.%20%3Ci%3EBulletin%20of%20the%20Museum%20of%20Comparative%20Zoology%3C%5C%2Fi%3E%2C%20%3Ci%3E162%3C%5C%2Fi%3E%281%29%2C%201%26%23x2013%3B58.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3099%5C%2FMCZ48.1%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3099%5C%2FMCZ48.1%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Boas%20of%20the%20World%20%28Superfamily%20Booidae%29%3A%20A%20Checklist%20With%20Systematic%2C%20Taxonomic%2C%20and%20Conservation%20Assessments%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Graham%22%2C%22lastName%22%3A%22Reynolds%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Robert%20W.%22%2C%22lastName%22%3A%22Henderson%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2209%5C%2F2018%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3099%5C%2FMCZ48.1%22%2C%22ISSN%22%3A%220027-4100%2C%201938-2987%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.bioone.org%5C%2Fdoi%5C%2F10.3099%5C%2FMCZ48.1%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-03T22%3A37%3A45Z%22%7D%7D%2C%7B%22key%22%3A%22LBFXPYAI%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Reynolds%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EReynolds%2C%20R.%20G.%2C%20Niemiller%2C%20M.%20L.%2C%20%26amp%3B%20Revell%2C%20L.%20J.%20%282014%29.%20Toward%20a%20Tree-of-Life%20for%20the%20boas%20and%20pythons%3A%20Multilocus%20species-level%20phylogeny%20with%20unprecedented%20taxon%20sampling.%20%3Ci%3EMolecular%20Phylogenetics%20and%20Evolution%3C%5C%2Fi%3E%2C%20%3Ci%3E71%3C%5C%2Fi%3E%2C%20201%26%23x2013%3B213.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2013.11.011%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2013.11.011%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Toward%20a%20Tree-of-Life%20for%20the%20boas%20and%20pythons%3A%20Multilocus%20species-level%20phylogeny%20with%20unprecedented%20taxon%20sampling%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20Graham%22%2C%22lastName%22%3A%22Reynolds%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthew%20L.%22%2C%22lastName%22%3A%22Niemiller%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Liam%20J.%22%2C%22lastName%22%3A%22Revell%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2202%5C%2F2014%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ympev.2013.11.011%22%2C%22ISSN%22%3A%2210557903%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1055790313004284%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-14T08%3A26%3A19Z%22%7D%7D%2C%7B%22key%22%3A%22Y7SA4KVW%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rogaev%20et%20al.%22%2C%22parsedDate%22%3A%222006-02-07%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ERogaev%2C%20E.%20I.%2C%20Moliaka%2C%20Y.%20K.%2C%20Malyarchuk%2C%20B.%20A.%2C%20Kondrashov%2C%20F.%20A.%2C%20Derenko%2C%20M.%20V.%2C%20Chumakov%2C%20I.%2C%20%26amp%3B%20Grigorenko%2C%20A.%20P.%20%282006%29.%20Complete%20Mitochondrial%20Genome%20and%20Phylogeny%20of%20Pleistocene%20Mammoth%20Mammuthus%20primigenius.%20%3Ci%3EPLoS%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E4%3C%5C%2Fi%3E%283%29%2C%20e73.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pbio.0040073%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pbio.0040073%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Complete%20Mitochondrial%20Genome%20and%20Phylogeny%20of%20Pleistocene%20Mammoth%20Mammuthus%20primigenius%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Evgeny%20I%22%2C%22lastName%22%3A%22Rogaev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuri%20K%22%2C%22lastName%22%3A%22Moliaka%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Boris%20A%22%2C%22lastName%22%3A%22Malyarchuk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fyodor%20A%22%2C%22lastName%22%3A%22Kondrashov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Miroslava%20V%22%2C%22lastName%22%3A%22Derenko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ilya%22%2C%22lastName%22%3A%22Chumakov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anastasia%20P%22%2C%22lastName%22%3A%22Grigorenko%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22David%22%2C%22lastName%22%3A%22Penny%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222006-2-7%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pbio.0040073%22%2C%22ISSN%22%3A%221545-7885%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pbio.0040073%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-10-24T10%3A47%3A31Z%22%7D%7D%2C%7B%22key%22%3A%22I7AINDKS%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Scanferla%20and%20Smith%22%2C%22parsedDate%22%3A%222020-03-13%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EScanferla%2C%20A.%2C%20%26amp%3B%20Smith%2C%20K.%20T.%20%282020%29.%20Exquisitely%20Preserved%20Fossil%20Snakes%20of%20Messel%3A%20Insight%20into%20the%20Evolution%2C%20Biogeography%2C%20Habitat%20Preferences%20and%20Sensory%20Ecology%20of%20Early%20Boas.%20%3Ci%3EDiversity%3C%5C%2Fi%3E%2C%20%3Ci%3E12%3C%5C%2Fi%3E%283%29%2C%20100.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fd12030100%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fd12030100%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Exquisitely%20Preserved%20Fossil%20Snakes%20of%20Messel%3A%20Insight%20into%20the%20Evolution%2C%20Biogeography%2C%20Habitat%20Preferences%20and%20Sensory%20Ecology%20of%20Early%20Boas%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Agust%5Cu00edn%22%2C%22lastName%22%3A%22Scanferla%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Krister%20T.%22%2C%22lastName%22%3A%22Smith%22%7D%5D%2C%22abstractNote%22%3A%22Our%20knowledge%20of%20early%20evolution%20of%20snakes%20is%20improving%2C%20but%20all%20that%20we%20can%20infer%20about%20the%20evolution%20of%20modern%20clades%20of%20snakes%20such%20as%20boas%20%28Booidea%29%20is%20still%20based%20on%20isolated%20bones.%20Here%2C%20we%20resolve%20the%20phylogenetic%20relationships%20of%20Eoconstrictor%20%5Cufb01scheri%20comb.%20nov.%20and%20other%20booids%20from%20the%20early-middle%20Eocene%20of%20Messel%20%28Germany%29%2C%20the%20best-known%20fossil%20snake%20assemblage%20yet%20discovered.%20Our%20combined%20analyses%20demonstrate%20an%20a%5Cufb03nity%20of%20Eoconstrictor%20with%20Neotropical%20boas%2C%20thus%20entailing%20a%20South%20America-to-Europe%20dispersal%20event.%20Other%20booid%20species%20from%20Messel%20are%20related%20to%20di%5Cufb00erent%20New%20World%20clades%2C%20reinforcing%20the%20cosmopolitan%20nature%20of%20the%20Messel%20booid%20fauna.%20Our%20analyses%20indicate%20that%20Eoconstrictor%20was%20a%20terrestrial%2C%20medium-%20to%20large-bodied%20snake%20that%20bore%20labial%20pit%20organs%20in%20the%20upper%20jaw%2C%20the%20earliest%20evidence%20that%20the%20visual%20system%20in%20snakes%20incorporated%20the%20infrared%20spectrum.%20Evaluation%20of%20the%20known%20palaeobiology%20of%20Eoconstrictor%20provides%20no%20evidence%20that%20pit%20organs%20played%20a%20role%20in%20the%20predator%5Cu2013prey%20relations%20of%20this%20stem%20boid.%20At%20the%20same%20time%2C%20the%20morphological%20diversity%20of%20Messel%20booids%20re%5Cufb02ects%20the%20occupation%20of%20several%20terrestrial%20macrohabitats%2C%20and%20even%20in%20the%20earliest%20booid%20community%20the%20relation%20between%20pit%20organs%20and%20body%20size%20is%20similar%20to%20that%20seen%20in%20booids%20today.%22%2C%22date%22%3A%222020-03-13%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fd12030100%22%2C%22ISSN%22%3A%221424-2818%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1424-2818%5C%2F12%5C%2F3%5C%2F100%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-09-24T21%3A06%3A40Z%22%7D%7D%2C%7B%22key%22%3A%22VC3XU4NX%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Scanlon%20and%20Lee%22%2C%22parsedDate%22%3A%222011%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EScanlon%2C%20J.%2C%20%26amp%3B%20Lee%2C%20M.%20%282011%29.%20%3Ci%3EThe%20Major%20Clades%20of%20Living%20Snakes%20Morphological%20Evolution%2C%20Molecular%20Phylogeny%20and%20Divergence%20Dates%3C%5C%2Fi%3E.%2055%26%23x2013%3B95.%20%5C%2Fz-wcorg%5C%2F.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Major%20Clades%20of%20Living%20Snakes%20Morphological%20Evolution%2C%20Molecular%20Phylogeny%20and%20Divergence%20Dates%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%22%2C%22lastName%22%3A%22Scanlon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Lee%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222011%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-26T00%3A14%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22MWAJWBEI%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Schwartz%20and%20Henderson%22%2C%22parsedDate%22%3A%221985%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ESchwartz%2C%20A.%2C%20%26amp%3B%20Henderson%2C%20R.%20W.%20%281985%29.%20%3Ci%3EA%20guide%20to%20the%20identification%20of%20the%20amphibians%20and%20reptiles%20of%20the%20West%20Indies%20exclusive%20of%20Hispaniola%3C%5C%2Fi%3E.%20Milwaukee%20Public%20Museum.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22A%20guide%20to%20the%20identification%20of%20the%20amphibians%20and%20reptiles%20of%20the%20West%20Indies%20exclusive%20of%20Hispaniola%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Albert%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Robert%20W.%22%2C%22lastName%22%3A%22Henderson%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221985%22%2C%22language%22%3A%22eng%22%2C%22ISBN%22%3A%22978-0-89326-112-2%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-09-16T14%3A19%3A59Z%22%7D%7D%2C%7B%22key%22%3A%22WPYXP4KX%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Sheplan%20and%20Schwartz%22%2C%22parsedDate%22%3A%221974%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ESheplan%2C%20B.%20R.%2C%20%26amp%3B%20Schwartz%2C%20A.%20%281974%29.%20Hispaniolan%20boas%20of%20the%20genus%20Epicrates%20%28Serpentes%2C%20Boidae%29%20and%20their%20Antillean%20relationships.%20%3Ci%3EAnnals%20of%20the%20Carnegie%20Museum%3C%5C%2Fi%3E%2C%20%3Ci%3E45%3C%5C%2Fi%3E%2C%2057%26%23x2013%3B143.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Hispaniolan%20boas%20of%20the%20genus%20Epicrates%20%28Serpentes%2C%20Boidae%29%20and%20their%20Antillean%20relationships.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B.R.%22%2C%22lastName%22%3A%22Sheplan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Schwartz%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221974%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-13T14%3A42%3A19Z%22%7D%7D%2C%7B%22key%22%3A%224WUMTCH6%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tolson%22%2C%22parsedDate%22%3A%221987%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ETolson%2C%20P.%20J.%20%281987%29.%20Phylogenetics%20of%20the%20boid%20snake%20genus%20Epicrates%20and%20Caribbean%20vicariance%20theory.%20%3Ci%3EOCCASIONAL%20PAPERS%20OF%20THE%20MUSEUM%20OF%20ZOOLOGY%20THE%20UNIVERSITY%20OF%20MICHIGAN%3C%5C%2Fi%3E%2C%20%3Ci%3E715%3C%5C%2Fi%3E%2C%201%26%23x2013%3B68.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdeepblue.lib.umich.edu%5C%2Fbitstream%5C%2Fhandle%5C%2F2027.42%5C%2F57151%5C%2FOP715.pdf%3Fsequence%3D1%26isAllowed%3Dy%27%3Ehttps%3A%5C%2F%5C%2Fdeepblue.lib.umich.edu%5C%2Fbitstream%5C%2Fhandle%5C%2F2027.42%5C%2F57151%5C%2FOP715.pdf%3Fsequence%3D1%26isAllowed%3Dy%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phylogenetics%20of%20the%20boid%20snake%20genus%20Epicrates%20and%20Caribbean%20vicariance%20theory%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peter%20J.%22%2C%22lastName%22%3A%22Tolson%22%7D%5D%2C%22abstractNote%22%3A%22http%3A%5C%2F%5C%2Fdeepblue.lib.umich.edu%5C%2Fbitstream%5C%2F2027.42%5C%2F57151%5C%2F1%5C%2FOP715.pdf%22%2C%22date%22%3A%221987%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdeepblue.lib.umich.edu%5C%2Fbitstream%5C%2Fhandle%5C%2F2027.42%5C%2F57151%5C%2FOP715.pdf%3Fsequence%3D1%26isAllowed%3Dy%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-22T22%3A04%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22VIMCTQSE%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tolson%20and%20Henderson%22%2C%22parsedDate%22%3A%222006%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3ETolson%2C%20P.%20J.%2C%20%26amp%3B%20Henderson%2C%20R.%20W.%20%282006%29.%20An%20overview%20of%20snake%20conservation%20in%20the%20West%20Indies.%20%3Ci%3EAPPLIED%20HERPETOLOGY%3C%5C%2Fi%3E%2C%20%3Ci%3E3%3C%5C%2Fi%3E%284%29%2C%20345%26%23x2013%3B356.%20%5C%2Fz-wcorg%5C%2F.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22An%20overview%20of%20snake%20conservation%20in%20the%20West%20Indies%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20J.%22%2C%22lastName%22%3A%22Tolson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%20W.%22%2C%22lastName%22%3A%22Henderson%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222006%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%221570-7539%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-02-28T10%3A03%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22P3X224ZR%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Viana%20et%20al.%22%2C%22parsedDate%22%3A%222016-08-05%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EViana%2C%20P.%20F.%2C%20Ribeiro%2C%20L.%20B.%2C%20Souza%2C%20G.%20M.%2C%20Chalkidis%2C%20H.%20de%20M.%2C%20Gross%2C%20M.%20C.%2C%20%26amp%3B%20Feldberg%2C%20E.%20%282016%29.%20Is%20the%20Karyotype%20of%20Neotropical%20Boid%20Snakes%20Really%20Conserved%3F%20Cytotaxonomy%2C%20Chromosomal%20Rearrangements%20and%20Karyotype%20Organization%20in%20the%20Boidae%20Family.%20%3Ci%3EPLOS%20ONE%3C%5C%2Fi%3E%2C%20%3Ci%3E11%3C%5C%2Fi%3E%288%29%2C%20e0160274.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0160274%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0160274%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Is%20the%20Karyotype%20of%20Neotropical%20Boid%20Snakes%20Really%20Conserved%3F%20Cytotaxonomy%2C%20Chromosomal%20Rearrangements%20and%20Karyotype%20Organization%20in%20the%20Boidae%20Family%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrik%20F.%22%2C%22lastName%22%3A%22Viana%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Leila%20B.%22%2C%22lastName%22%3A%22Ribeiro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22George%20Myller%22%2C%22lastName%22%3A%22Souza%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hip%5Cu00f3crates%20de%20Menezes%22%2C%22lastName%22%3A%22Chalkidis%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maria%20Claudia%22%2C%22lastName%22%3A%22Gross%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eliana%22%2C%22lastName%22%3A%22Feldberg%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Kunbo%22%2C%22lastName%22%3A%22Wang%22%7D%5D%2C%22abstractNote%22%3A%22Boids%20are%20primitive%20snakes%20from%20a%20basal%20lineage%20that%20is%20widely%20distributed%20in%20Neotropical%20region.%20Many%20of%20these%20species%20are%20both%20morphologically%20and%20biogeographically%20divergent%2C%20and%20the%20relationship%20among%20some%20species%20remains%20uncertain%20even%20with%20evolutionary%20and%20phylogenetic%20studies%20being%20proposed%20for%20the%20group.%20For%20a%20better%20understanding%20of%20the%20evolutionary%20relationship%20between%20these%20snakes%2C%20we%20cytogenetically%20analysed%207%20species%20and%203%20subspecies%20of%20Neotropical%20snakes%20from%20the%20Boidae%20family%20using%20different%20chromosomal%20markers.%20The%20karyotypes%20of%20Boa%20constrictor%20occidentalis%2C%20Corallus%20hortulanus%2C%20Eunectes%20notaeus%2C%20Epicrates%20cenchria%20and%20Epicrates%20assisi%20are%20presented%20here%20for%20the%20first%20time%20with%20the%20redescriptions%20of%20the%20karyotypes%20of%20Boa%20constrictor%20constrictor%2C%20B.%20c.%20amarali%2C%20Eunectes%20murinus%20and%20Epicrates%20crassus.%20The%20three%20subspecies%20of%20Boa%2C%20two%20species%20of%20Eunectes%20and%20three%20species%20of%20Epicrates%20exhibit%202n%20%3D%2036%20chromosomes.%20In%20contrast%2C%20C.%20hortulanus%20presented%20a%20totally%20different%20karyotype%20composition%20for%20the%20Boidae%20family%2C%20showing%202n%20%3D%2040%20chromosomes%20with%20a%20greater%20number%20of%20macrochromosomes.%20Furthermore%2C%20chromosomal%20mapping%20of%20telomeric%20sequences%20revealed%20the%20presence%20of%20interstitial%20telomeric%20sites%20%28ITSs%29%20on%20many%20chromosomes%20in%20addition%20to%20the%20terminal%20markings%20on%20all%20chromosomes%20of%20all%20taxa%20analysed%2C%20with%20the%20exception%20of%20E.%20notaeus.%20Thus%2C%20we%20demonstrate%20that%20the%20karyotypes%20of%20these%20snakes%20are%20not%20as%20highly%20conserved%20as%20previously%20thought.%20Moreover%2C%20we%20provide%20an%20overview%20of%20the%20current%20cytotaxonomy%20of%20the%20group.%22%2C%22date%22%3A%222016-8-5%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0160274%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pone.0160274%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-16T23%3A07%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22SHGCJE3C%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Viana%20et%20al.%22%2C%22parsedDate%22%3A%222020-10-10%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EViana%2C%20P.%20F.%2C%20Ezaz%2C%20T.%2C%20Cioffi%2C%20M.%20de%20B.%2C%20Liehr%2C%20T.%2C%20Al-Rikabi%2C%20A.%2C%20Tavares-Pinheiro%2C%20R.%2C%20Bertollo%2C%20L.%20A.%20C.%2C%20%26amp%3B%20Feldberg%2C%20E.%20%282020%29.%20Revisiting%20the%20Karyotype%20Evolution%20of%20Neotropical%20Boid%20Snakes%3A%20A%20Puzzle%20Mediated%20by%20Chromosomal%20Fissions.%20%3Ci%3ECells%3C%5C%2Fi%3E%2C%20%3Ci%3E9%3C%5C%2Fi%3E%2810%29%2C%202268.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcells9102268%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcells9102268%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Revisiting%20the%20Karyotype%20Evolution%20of%20Neotropical%20Boid%20Snakes%3A%20A%20Puzzle%20Mediated%20by%20Chromosomal%20Fissions%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrik%20F.%22%2C%22lastName%22%3A%22Viana%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tariq%22%2C%22lastName%22%3A%22Ezaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marcelo%20de%20Bello%22%2C%22lastName%22%3A%22Cioffi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Liehr%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ahmed%22%2C%22lastName%22%3A%22Al-Rikabi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rodrigo%22%2C%22lastName%22%3A%22Tavares-Pinheiro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luiz%20Ant%5Cu00f4nio%20Carlos%22%2C%22lastName%22%3A%22Bertollo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eliana%22%2C%22lastName%22%3A%22Feldberg%22%7D%5D%2C%22abstractNote%22%3A%22The%20Boidae%20family%20is%20an%20ancient%20group%20of%20snakes%20widely%20distributed%20across%20the%20Neotropical%20region%2C%20where%20several%20biogeographic%20events%20contributed%20towards%20shaping%20their%20evolution%20and%20diversi%5Cufb01cation.%20Most%20species%20of%20this%20family%20have%20a%20diploid%20number%20composed%20of%202n%20%3D%2036%3B%20however%2C%20among%20Booidea%20families%2C%20the%20Boidae%20stands%20out%20by%20presenting%20the%20greatest%20chromosomal%20diversity%2C%20with%202n%20ranging%20between%2036%20and%2044%20chromosomes%20and%20an%20undi%5Cufb00erentiated%20XY%20sex%20chromosome%20system.%20Here%2C%20we%20applied%20a%20comparative%20chromosome%20analysis%20using%20cross-species%20chromosome%20paintings%20in%20%5Cufb01ve%20species%20representing%20four%20Boidae%20genera%2C%20to%20decipher%20the%20evolutionary%20dynamics%20of%20some%20chromosomes%20in%20these%20Neotropical%20snakes.%20Our%20study%20included%20all%20diploid%20numbers%20%282n%20%3D%2036%2C%2040%2C%20and%2044%29%20known%20for%20this%20family%20and%20our%20comparative%20chromosomal%20mappings%20point%20to%20a%20strong%20evolutionary%20relationship%20among%20the%20genera%20Boa%2C%20Corallus%2C%20Eunectes%2C%20and%20Epicrates.%20The%20results%20also%20allowed%20us%20to%20propose%20the%20cytogenomic%20diversi%5Cufb01cation%20that%20had%20occurred%20in%20this%20family%3A%20a%20process%20mediated%20by%20centric%20%5Cufb01ssions%2C%20including%20%5Cufb01ssion%20events%20of%20the%20putative%20and%20undi%5Cufb00erentiated%20XY%20sex%20chromosome%20system%20in%20the%202n%20%3D%2044%20karyotype%2C%20which%20is%20critical%20in%20solving%20the%20puzzle%20of%20the%20karyotype%20evolution%20of%20boid%20snakes.%22%2C%22date%22%3A%222020-10-10%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fcells9102268%22%2C%22ISSN%22%3A%222073-4409%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2073-4409%5C%2F9%5C%2F10%5C%2F2268%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-10-22T19%3A51%3A25Z%22%7D%7D%2C%7B%22key%22%3A%226I2NMGSM%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Vicoso%20et%20al.%22%2C%22parsedDate%22%3A%222013-08-27%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EVicoso%2C%20B.%2C%20Emerson%2C%20J.%20J.%2C%20Zektser%2C%20Y.%2C%20Mahajan%2C%20S.%2C%20%26amp%3B%20Bachtrog%2C%20D.%20%282013%29.%20Comparative%20Sex%20Chromosome%20Genomics%20in%20Snakes%3A%20Differentiation%2C%20Evolutionary%20Strata%2C%20and%20Lack%20of%20Global%20Dosage%20Compensation.%20%3Ci%3EPLoS%20Biology%3C%5C%2Fi%3E%2C%20%3Ci%3E11%3C%5C%2Fi%3E%288%29%2C%20e1001643.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pbio.1001643%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pbio.1001643%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Comparative%20Sex%20Chromosome%20Genomics%20in%20Snakes%3A%20Differentiation%2C%20Evolutionary%20Strata%2C%20and%20Lack%20of%20Global%20Dosage%20Compensation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beatriz%22%2C%22lastName%22%3A%22Vicoso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20J.%22%2C%22lastName%22%3A%22Emerson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yulia%22%2C%22lastName%22%3A%22Zektser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shivani%22%2C%22lastName%22%3A%22Mahajan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Doris%22%2C%22lastName%22%3A%22Bachtrog%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Nick%20H.%22%2C%22lastName%22%3A%22Barton%22%7D%5D%2C%22abstractNote%22%3A%22Snakes%20exhibit%20genetic%20sex%20determination%2C%20with%20female%20heterogametic%20sex%20chromosomes%20%28ZZ%20males%2C%20ZW%20females%29.%20Extensive%20cytogenetic%20work%20has%20suggested%20that%20the%20level%20of%20sex%20chromosome%20heteromorphism%20varies%20among%20species%2C%20with%20Boidae%20having%20entirely%20homomorphic%20sex%20chromosomes%2C%20Viperidae%20having%20completely%20heteromorphic%20sex%20chromosomes%2C%20and%20Colubridae%20showing%20partial%20differentiation.%20Here%2C%20we%20take%20a%20genomic%20approach%20to%20compare%20sex%20chromosome%20differentiation%20in%20these%20three%20snake%20families.%20We%20identify%20homomorphic%20sex%20chromosomes%20in%20boas%20%28Boidae%29%2C%20but%20completely%20heteromorphic%20sex%20chromosomes%20in%20both%20garter%20snakes%20%28Colubridae%29%20and%20pygmy%20rattlesnake%20%28Viperidae%29.%20Detection%20of%20W-linked%20gametologs%20enables%20us%20to%20establish%20the%20presence%20of%20evolutionary%20strata%20on%20garter%20and%20pygmy%20rattlesnake%20sex%20chromosomes%20where%20recombination%20was%20abolished%20at%20different%20time%20points.%20Sequence%20analysis%20shows%20that%20all%20strata%20are%20shared%20between%20pygmy%20rattlesnake%20and%20garter%20snake%2C%20i.e.%2C%20recombination%20was%20abolished%20between%20the%20sex%20chromosomes%20before%20the%20two%20lineages%20diverged.%20The%20sex-biased%20transmission%20of%20the%20Z%20and%20its%20hemizygosity%20in%20females%20can%20impact%20patterns%20of%20molecular%20evolution%2C%20and%20we%20show%20that%20rates%20of%20evolution%20for%20Z-linked%20genes%20are%20increased%20relative%20to%20their%20pseudoautosomal%20homologs%2C%20both%20at%20synonymous%20and%20amino%20acid%20sites%20%28even%20after%20controlling%20for%20mutational%20biases%29.%20This%20demonstrates%20that%20mutation%20rates%20are%20male-biased%20in%20snakes%20%28male-driven%20evolution%29%2C%20but%20also%20supports%20faster-Z%20evolution%20due%20to%20differential%20selective%20effects%20on%20the%20Z.%20Finally%2C%20we%20perform%20a%20transcriptome%20analysis%20in%20boa%20and%20pygmy%20rattlesnake%20to%20establish%20baseline%20levels%20of%20sex-biased%20expression%20in%20homomorphic%20sex%20chromosomes%2C%20and%20show%20that%20heteromorphic%20ZW%20chromosomes%20in%20rattlesnakes%20lack%20chromosome-wide%20dosage%20compensation.%20Our%20study%20provides%20the%20first%20full%20scale%20overview%20of%20the%20evolution%20of%20snake%20sex%20chromosomes%20at%20the%20genomic%20level%2C%20thus%20greatly%20expanding%20our%20knowledge%20of%20reptilian%20and%20vertebrate%20sex%20chromosomes%20evolution.%22%2C%22date%22%3A%222013-8-27%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pbio.1001643%22%2C%22ISSN%22%3A%221545-7885%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pbio.1001643%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222020-12-04T18%3A26%3A06Z%22%7D%7D%2C%7B%22key%22%3A%22E7QA9IZV%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Vidal%20and%20Hedges%22%2C%22parsedDate%22%3A%222009%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EVidal%2C%20N.%2C%20%26amp%3B%20Hedges%2C%20S.%20B.%20%282009%29.%20The%20molecular%20evolutionary%20tree%20of%20lizards%2C%20snakes%2C%20and%20amphisbaenians.%20%3Ci%3EComptes%20Rendus%20Biologies%3C%5C%2Fi%3E%2C%20%3Ci%3E332%3C%5C%2Fi%3E%282%26%23x2013%3B3%29%2C%20129%26%23x2013%3B139.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.crvi.2008.07.010%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.crvi.2008.07.010%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20molecular%20evolutionary%20tree%20of%20lizards%2C%20snakes%2C%20and%20amphisbaenians%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Vidal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%20Blair%22%2C%22lastName%22%3A%22Hedges%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222%5C%2F2009%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.crvi.2008.07.010%22%2C%22ISSN%22%3A%2216310691%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS163106910800190X%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-24T13%3A21%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22K7ZY4IFP%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Vidal%20and%20Hedges%22%2C%22parsedDate%22%3A%222002%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EVidal%2C%20N.%2C%20%26amp%3B%20Hedges%2C%20S.%20B.%20%282002%29.%20Higher-level%20relationships%20of%20snakes%20inferred%20from%20four%20nuclear%20and%20mitochondrial%20genes.%20%3Ci%3EComptes%20Rendus%20Biologies%3C%5C%2Fi%3E%2C%20%3Ci%3E325%3C%5C%2Fi%3E%289%29%2C%20977%26%23x2013%3B985.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS1631-0691%2802%2901510-X%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS1631-0691%2802%2901510-X%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Higher-level%20relationships%20of%20snakes%20inferred%20from%20four%20nuclear%20and%20mitochondrial%20genes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Vidal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.Blair%22%2C%22lastName%22%3A%22Hedges%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%229%5C%2F2002%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2FS1631-0691%2802%2901510-X%22%2C%22ISSN%22%3A%2216310691%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS163106910201510X%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-03-26T00%3A14%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22MEBZP7NS%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Vitt%20et%20al.%22%2C%22parsedDate%22%3A%222009%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EVitt%2C%20L.%20J.%2C%20Caldwell%2C%20J.%20P.%2C%20%26amp%3B%20Zug%2C%20G.%20R.%20%282009%29.%20%3Ci%3EHerpetology%3C%5C%2Fi%3E%20%283rd%20Edition%2C%20completely%20revised%20and%20expanded%29.%20Elsevier%5C%2FAP%2C%20Academic%20Press%20is%20an%20imprint%20of%20Elsevier.%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22Herpetology%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurie%20J.%22%2C%22lastName%22%3A%22Vitt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Janalee%20P.%22%2C%22lastName%22%3A%22Caldwell%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22George%20R.%22%2C%22lastName%22%3A%22Zug%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222009%22%2C%22language%22%3A%22%22%2C%22ISBN%22%3A%22978-0-12-374346-6%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-06T11%3A12%3A17Z%22%7D%7D%2C%7B%22key%22%3A%22P77ZY57F%22%2C%22library%22%3A%7B%22id%22%3A2129430%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zheng%20and%20Wiens%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%3EZheng%2C%20Y.%2C%20%26amp%3B%20Wiens%2C%20J.%20J.%20%282016%29.%20Combining%20phylogenomic%20and%20supermatrix%20approaches%2C%20and%20a%20time-calibrated%20phylogeny%20for%20squamate%20reptiles%20%28lizards%20and%20snakes%29%20based%20on%2052%20genes%20and%204162%20species.%20%3Ci%3EMolecular%20Phylogenetics%20and%20Evolution%3C%5C%2Fi%3E%2C%20%3Ci%3E94%3C%5C%2Fi%3E%2C%20537%26%23x2013%3B547.%20%3Ca%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2015.10.009%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ympev.2015.10.009%3C%5C%2Fa%3E%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Combining%20phylogenomic%20and%20supermatrix%20approaches%2C%20and%20a%20time-calibrated%20phylogeny%20for%20squamate%20reptiles%20%28lizards%20and%20snakes%29%20based%20on%2052%20genes%20and%204162%20species%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuchi%22%2C%22lastName%22%3A%22Zheng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20J.%22%2C%22lastName%22%3A%22Wiens%22%7D%5D%2C%22abstractNote%22%3A%22Two%20common%20approaches%20for%20estimating%20phylogenies%20in%20species-rich%20groups%20are%20to%3A%20%28i%29%20sample%20many%20loci%20for%20few%20species%20%28e.g.%20phylogenomic%20approach%29%2C%20or%20%28ii%29%20sample%20many%20species%20for%20fewer%20loci%20%28e.g.%20supermatrix%20approach%29.%20In%20theory%2C%20these%20approaches%20can%20be%20combined%20to%20simultaneously%20resolve%20both%20higher-level%20relationships%20%28with%20many%20genes%29%20and%20species-level%20relationships%20%28with%20many%20taxa%29.%20However%2C%20fundamental%20questions%20remain%20unanswered%20about%20this%20combined%20approach.%20First%2C%20will%20higher-level%20relationships%20more%20closely%20resemble%20those%20estimated%20from%20many%20genes%20or%20those%20from%20many%20taxa%3F%20Second%2C%20will%20branch%20support%20increase%20for%20higher-level%20relationships%20%28relative%20to%20the%20estimate%20from%20many%20taxa%29%3F%20Here%2C%20we%20address%20these%20questions%20in%20squamate%20reptiles.%20We%20combined%20two%20recently%20published%20datasets%2C%20one%20based%20on%2044%20genes%20for%20161%20species%2C%20and%20one%20based%20on%2012%20genes%20for%204161%20species.%20The%20likelihood-based%20tree%20from%20the%20combined%20matrix%20%2852%20genes%2C%204162%20species%29%20shared%20more%20higher-level%20clades%20with%20the%2044-gene%20tree%20%2890%25%20vs.%2077%25%20shared%29.%20Branch%20support%20for%20higher%20level-relationships%20was%20marginally%20higher%20than%20in%20the%2012-gene%20tree%2C%20but%20lower%20than%20in%20the%2044-gene%20tree.%20Relationships%20were%20apparently%20not%20obscured%20by%20the%20abundant%20missing%20data%20%2892%25%20overall%29.%20We%20provide%20a%20time-calibrated%20phylogeny%20based%20on%20extensive%20sampling%20of%20genes%20and%20taxa%20as%20a%20resource%20for%20comparative%20studies.%22%2C%22date%22%3A%2201%5C%2F2016%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ympev.2015.10.009%22%2C%22ISSN%22%3A%2210557903%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1055790315003127%22%2C%22collections%22%3A%5B%224E2FHAKS%22%5D%2C%22dateModified%22%3A%222018-09-03T22%3A37%3A45Z%22%7D%7D%5D%7D Bogert, C. M. (1968). The variations and affinities of the dwarf boas of the genus Ungaliophis. AMERICAN MUSEUM NOVITATES , 2340 , 1–26. /z-wcorg/.
Burbrink, F. T., & Pyron, R. A. (2008). The Taming of the Skew: Estimating Proper Confidence Intervals for Divergence Dates.
Systematic Biology ,
57 (2), 317–328.
https://doi.org/10.1080/10635150802040605 Esquerré, D., & Keogh, J. S. (2016). Parallel selective pressures drive convergent diversification of phenotypes in pythons and boas.
Ecology Letters ,
19 (7), 800–809.
https://doi.org/10.1111/ele.12620 Gamble, T., Castoe, T. A., Nielsen, S. V., Banks, J. L., Card, D. C., Schield, D. R., Schuett, G. W., & Booth, W. (2017). The Discovery of XY Sex Chromosomes in a Boa and Python.
Current Biology ,
27 (14), 2148-2153.e4.
https://doi.org/10.1016/j.cub.2017.06.010 Gilbert, M. T. P., Drautz, D. I., Lesk, A. M., Ho, S. Y. W., Qi, J., Ratan, A., Hsu, C.-H., Sher, A., Dalen, L., Gotherstrom, A., Tomsho, L. P., Rendulic, S., Packard, M., Campos, P. F., Kuznetsova, T. V., Shidlovskiy, F., Tikhonov, A., Willerslev, E., Iacumin, P., … Schuster, S. C. (2008). Intraspecific phylogenetic analysis of Siberian woolly mammoths using complete mitochondrial genomes.
Proceedings of the National Academy of Sciences ,
105 (24), 8327–8332.
https://doi.org/10.1073/pnas.0802315105 Guo, P., Liu, Q., Xu, Y., Jiang, K., Hou, M., Ding, L., Pyron, R. A., & Burbrink, F. T. (2012). Out of Asia: Natricine snakes support the Cenozoic Beringian Dispersal Hypothesis.
Molecular Phylogenetics and Evolution ,
63 (3), 825–833.
https://doi.org/10.1016/j.ympev.2012.02.021 Hsiang, A. Y., Field, D. J., Webster, T. H., Behlke, A. D., Davis, M. B., Racicot, R. A., & Gauthier, J. A. (2015). The origin of snakes: revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record.
BMC Evolutionary Biology ,
15 (1), 87.
https://doi.org/10.1186/s12862-015-0358-5 Lee, M. S. Y., Hugall, A. F., Lawson, R., & Scanlon, J. D. (2007). Phylogeny of snakes (Serpentes): Combining morphological and molecular data in likelihood, Bayesian and parsimony analyses.
Systematics and Biodiversity ,
5 (4), 371–389.
https://doi.org/10.1017/S1477200007002290 Matsubara, K., Tarui, H., Toriba, M., Yamada, K., Nishida-Umehara, C., Agata, K., & Matsuda, Y. (2006). Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes.
Proceedings of the National Academy of Sciences of the United States of America ,
103 (48), 18190–18195.
www.pnas.org/cgi/doi/10.1073/pnas.0605274103 Nellis, D. W., Norton, R. L., & MacLean, W. P. (1983). On the Biogeography of the Virgin Islands Boa, Epicrates monensis granti.
Journal of Herpetology ,
17 (4), 413.
https://doi.org/10.2307/1563600 Noonan, J. P. (2010). Neanderthal genomics and the evolution of modern humans.
Genome Research ,
20 (5), 547–553.
https://doi.org/10.1101/gr.076000.108 Noonan, J. P. (2005). Genomic Sequencing of Pleistocene Cave Bears.
Science ,
309 (5734), 597–599.
https://doi.org/10.1126/science.1113485 Pyron, R. A., Reynolds, R. G., & Burbrink, F. T. (2014). A Taxonomic Revision of Boas (Serpentes: Boidae).
Zootaxa ,
3846 (2), 249.
https://doi.org/10.11646/zootaxa.3846.2.5 Pyron, R. A., Burbrink, F. T., & Wiens, J. J. (2013). A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes.
BMC Evolutionary Biology ,
13 (1), 93.
https://doi.org/10.1186/1471-2148-13-93 Reiserer, R., Schuett, G., & Beck, D. (2013). Taxonomic reassessment and conservation status of the beaded lizard, Heloderma horridum (Squamata: Helodermatidae). Amphibian & Reptile Conservation , 7 , 74–96.
Reynolds, R. G., & Henderson, R. W. (2018). Boas of the World (Superfamily Booidae): A Checklist With Systematic, Taxonomic, and Conservation Assessments.
Bulletin of the Museum of Comparative Zoology ,
162 (1), 1–58.
https://doi.org/10.3099/MCZ48.1 Reynolds, R. G., Niemiller, M. L., & Revell, L. J. (2014). Toward a Tree-of-Life for the boas and pythons: Multilocus species-level phylogeny with unprecedented taxon sampling.
Molecular Phylogenetics and Evolution ,
71 , 201–213.
https://doi.org/10.1016/j.ympev.2013.11.011 Rogaev, E. I., Moliaka, Y. K., Malyarchuk, B. A., Kondrashov, F. A., Derenko, M. V., Chumakov, I., & Grigorenko, A. P. (2006). Complete Mitochondrial Genome and Phylogeny of Pleistocene Mammoth Mammuthus primigenius.
PLoS Biology ,
4 (3), e73.
https://doi.org/10.1371/journal.pbio.0040073 Scanferla, A., & Smith, K. T. (2020). Exquisitely Preserved Fossil Snakes of Messel: Insight into the Evolution, Biogeography, Habitat Preferences and Sensory Ecology of Early Boas.
Diversity ,
12 (3), 100.
https://doi.org/10.3390/d12030100 Scanlon, J., & Lee, M. (2011). The Major Clades of Living Snakes Morphological Evolution, Molecular Phylogeny and Divergence Dates . 55–95. /z-wcorg/.
Schwartz, A., & Henderson, R. W. (1985). A guide to the identification of the amphibians and reptiles of the West Indies exclusive of Hispaniola . Milwaukee Public Museum.
Sheplan, B. R., & Schwartz, A. (1974). Hispaniolan boas of the genus Epicrates (Serpentes, Boidae) and their Antillean relationships. Annals of the Carnegie Museum , 45 , 57–143.
Tolson, P. J. (1987). Phylogenetics of the boid snake genus Epicrates and Caribbean vicariance theory.
OCCASIONAL PAPERS OF THE MUSEUM OF ZOOLOGY THE UNIVERSITY OF MICHIGAN ,
715 , 1–68.
https://deepblue.lib.umich.edu/bitstream/handle/2027.42/57151/OP715.pdf?sequence=1&isAllowed=y Tolson, P. J., & Henderson, R. W. (2006). An overview of snake conservation in the West Indies. APPLIED HERPETOLOGY , 3 (4), 345–356. /z-wcorg/.
Viana, P. F., Ribeiro, L. B., Souza, G. M., Chalkidis, H. de M., Gross, M. C., & Feldberg, E. (2016). Is the Karyotype of Neotropical Boid Snakes Really Conserved? Cytotaxonomy, Chromosomal Rearrangements and Karyotype Organization in the Boidae Family.
PLOS ONE ,
11 (8), e0160274.
https://doi.org/10.1371/journal.pone.0160274 Viana, P. F., Ezaz, T., Cioffi, M. de B., Liehr, T., Al-Rikabi, A., Tavares-Pinheiro, R., Bertollo, L. A. C., & Feldberg, E. (2020). Revisiting the Karyotype Evolution of Neotropical Boid Snakes: A Puzzle Mediated by Chromosomal Fissions.
Cells ,
9 (10), 2268.
https://doi.org/10.3390/cells9102268 Vicoso, B., Emerson, J. J., Zektser, Y., Mahajan, S., & Bachtrog, D. (2013). Comparative Sex Chromosome Genomics in Snakes: Differentiation, Evolutionary Strata, and Lack of Global Dosage Compensation.
PLoS Biology ,
11 (8), e1001643.
https://doi.org/10.1371/journal.pbio.1001643 Vidal, N., & Hedges, S. B. (2009). The molecular evolutionary tree of lizards, snakes, and amphisbaenians.
Comptes Rendus Biologies ,
332 (2–3), 129–139.
https://doi.org/10.1016/j.crvi.2008.07.010 Vidal, N., & Hedges, S. B. (2002). Higher-level relationships of snakes inferred from four nuclear and mitochondrial genes.
Comptes Rendus Biologies ,
325 (9), 977–985.
https://doi.org/10.1016/S1631-0691(02)01510-X Vitt, L. J., Caldwell, J. P., & Zug, G. R. (2009). Herpetology (3rd Edition, completely revised and expanded). Elsevier/AP, Academic Press is an imprint of Elsevier.
Zheng, Y., & Wiens, J. J. (2016). Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species.
Molecular Phylogenetics and Evolution ,
94 , 537–547.
https://doi.org/10.1016/j.ympev.2015.10.009